Abstract
Introduction
The JAVELIN Bladder 100 trial showed that maintenance avelumab therapy after chemotherapy improved the survival of patients with advanced or metastatic urothelial carcinoma. We analyzed the cost-effectiveness of maintenance therapy with avelumab plus best supportive care (BSC) in patients with advanced or metastatic urothelial carcinoma after receiving first-line platinum-based chemotherapy from the US payer perspective.
Methods
A Markov model was used to analyze the economic outcomes of maintenance avelumab plus BSC (avelumab strategy) in the treatment of urothelial carcinoma. The clinical data were derived from the JAVELIN Bladder 100 trial. All cost information was obtained from Medicare and published literature. The total cost, total life years (LYs), total quality-adjusted LYs (QALYs), incremental cost-effectiveness ratio (ICER), and incremental net health benefit (INHB) were calculated. One-way sensitivity analysis and probabilistic sensitivity analysis were also performed.
Results
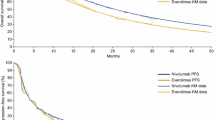
Our results showed that avelumab strategy versus BSC strategy cost US $176,352 and $238,661 and yielded an additional 0.465 and 1.007 QALY in all patients with unknown programmed-death ligand 1 (PD-L1) status and the PD-L1-positive subpopulation, respectively, which led to an ICER of $102,365/QALY and $106,253/QALY gained. In all patients with unknown PD-L1 status, maintenance avelumab plus BSC therapy guiding by PD-L1 expression testing (PD-L1-guided strategy) compared with the avelumab strategy and BSC strategy resulted in ICER of $105,360/QALY and $122,653/QALY, respectively. The probabilities of the avelumab strategy and the PD-L1-guided strategy being cost-effective in the simultaneous competition of the three strategies were 38.49% and 48.82%. In patients with PD-L1-positive status, the avelumab strategy had an 87.51% probability of cost-effectiveness. The most influential parameter for the model was the cost of avelumab and pembrolizumab.
Conclusions
This analysis demonstrated that maintenance therapy with avelumab plus BSC may be a cost-effective option for patients with advanced or metastatic urothelial carcinoma at a willingness-to-pay (WTP) threshold of $150,000/QALY, especially for patients with PD-L1-positive status.



Similar content being viewed by others
References
National Institutes of Health; National Cancer Institute; Surveillance, Epidemiology, and End Results Program. Cancer stat facts: bladder cancer. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed 14 Apr 2021.
Criss SD, Weaver DT, Sheehan DF, et al. Effect of PD-L1 testing on the cost-effectiveness and budget impact of pembrolizumab for advanced urothelial carcinoma of the bladder in the United States. Urol Oncol. 2019;37(3):180e111–8.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
National Comprehensive Cancer Network. Clinical practice guidelines in oncology: bladder cancer, version 2. 2021. https://www.nccn.org/professionals/physician_gls/PDF/bladder.pdf. Accessed 10 July 2021.
Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–30.
Grivas P, Monk BJ, Petrylak D, et al. Immune checkpoint inhibitors as switch or continuation maintenance therapy in solid tumors: rationale and current state. Target Oncol. 2019;14(5):505–25.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9.
Arias E, Xu J. United States life tables, 2018. Natl Vital Stat Rep. 2020;69(12):1–45.
US Department of Labor. Calculators. https://www.bls.gov/data/inflation_calculator.htm. Accessed 10 Apr 2021.
US: ASP Drug Pricing Files. Centers for Medicare and Medicaid Services. 2021. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2021-asp-drug-pricing-files. Accessed Apr 2021.
US: Centers for Medicare and Medicaid Services. 2021 Medicare physician fee schedule. 2021. https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed 26 Apr 2021.
Hale O, Patterson K, Lai Y, et al. Cost-effectiveness of pembrolizumab versus carboplatin-based chemotherapy as first-line treatment of PD-L1-positive locally advanced or metastatic urothelial carcinoma ineligible for cisplatin-based therapy in the United States. Clin Genitourin Cancer. 2021;19(1):e17–30.
Stevenson SM, Danzig MR, Ghandour RA, et al. Cost-effectiveness of neoadjuvant chemotherapy before radical cystectomy for muscle-invasive bladder cancer. Urol Oncol. 2014;32(8):1172–7.
Patterson K, Prabhu V, Xu R, et al. Cost-effectiveness of pembrolizumab for patients with advanced, unresectable, or metastatic urothelial cancer ineligible for cisplatin-based therapy. Eur Urol Oncol. 2019;2(5):565–71.
Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103.
Institute for Clinical and Economic Review Releases Final Value Assessment Framework for 2017–2019. https://icer.org/news-insights/press-releases/vaf-update-2017-2019/. Accessed 15 Mar 2021.
Wu B, Ma F. Cost-effectiveness of adding atezolizumab to first-line chemotherapy in patients with advanced triple-negative breast cancer. Ther Adv Med Oncol. 2020;12:1758835920916000.
Uyl-de Groot CA, Lowenberg B. Sustainability and affordability of cancer drugs: a novel pricing model. Nat Rev Clin Oncol. 2018;15(7):405–6.
Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14(12):1165–74.
Qin S, Yi L, Li S, et al. Cost-effectiveness of atezolizumab plus chemotherapy as first-line therapy for metastatic urothelial cancer. Adv Ther. 2021;38:3399–3408.
Bullement A, Nathan P, Willis A, et al. Cost effectiveness of avelumab for metastatic Merkel cell carcinoma. Pharmacoecon Open. 2019;3(3):377–90.
Chang WC, Lin AY, Hsu JC, et al. A cost-utility analysis of avelumab for metastatic Merkel cell carcinoma in Taiwan. Cancer Rep (Hoboken). 2021;e1399. https://doi.org/10.1002/cnr2.1399.
Lu P, Liang W, Li J, et al. A cost-effectiveness analysis: first-line avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. Front Pharmacol. 2020;11:619.
Acknowledgements
Funding
The work was supported by grants from the National Natural Science Foundation of China (Grant numbers 82073818 and 71874209); and the Key Science-Technology Research and Development Program of Hunan Province (Grant number 2020JJ8046); and the Hunan Provincial Natural Science Foundation of China (Grant number 2019JJ40411). The journal’s Rapid Service Fee was funded by the study sponsor.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Study design and supervision: Xiaomin Wan and Chongqing Tan; Data analysis and interpretation: Sini Li, Lidan Yi, Xia Luo; Data collection: Liubao Peng, Liting Wang, Shuxia Qin, Qiao Liu; Manuscript writing: Ye Peng and Zhihua She; Final approved of manuscript: All authors.
Disclosures
Ye Peng, Zhihua She, Liubao Peng, Qiao Liu, Lidan Yi, Xia Luo, Sini Li, Liting Wang, Shuxia Qin, Xiaomin Wan and Chongqing Tan confirm they have no conflicts of interest to declare.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peng, Y., She, Z., Peng, L. et al. Cost-Effectiveness of Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma in the United States. Adv Ther 38, 5710–5720 (2021). https://doi.org/10.1007/s12325-021-01950-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01950-0