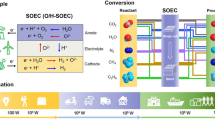
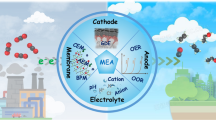
Abstract
The adverse effects of global warming and climate change have driven the exploration of feasible routes for CO2 capture, storage, conversion and utilization. The processes related to CO2 conversion in high-temperature electrochemical devices (HTEDs) using dense ceramic membranes are particularly appealing due to the simultaneous realization of highly efficient CO2 conversion and value-added chemical production as well as the generation of electricity and storage of renewable energy in some cases. Currently, most studies are focused on the two processes, CO2 electrolysis and H2O/CO2 co-electrolysis in oxygen-conducting solid oxide electrolysis cell (O-SOEC) reactors. Less attention has been paid to other meaningful CO2-conversion-related processes in HTEDs and systematic summary and analysis are currently not available. This review will fill the gap and classify the CO2-conversion-related processes in HTEDs reported in recent years into four types according to the related reactions, including assisted CO2 reduction to CO, H2O and CO2 co-conversion, dry reforming of methane and CO2 hydrogenation. Firstly, an overview of the fundamentals of HTED processes is presented, and then the related mechanism and research progress of each type of reactions in different HTEDs are elucidated and concluded accordingly. The remaining major technical issues are also briefly introduced. Lastly, the main challenges and feasible solutions as well as the future prospects of HTEDs for CO2-conversion-related processes are also discussed in this review.
Graphical Abstract






















Similar content being viewed by others
Change history
19 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s41918-021-00123-5
References
Kondratenko, E.V., Mul, G., Baltrusaitis, J., et al.: Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 6, 3112–3135 (2013). https://doi.org/10.1039/c3ee41272e
Wang, C.L., Sun, Z.X., Zheng, Y., et al.: Recent progress in visible light photocatalytic conversion of carbon dioxide. J. Mater. Chem. A 7, 865–887 (2019). https://doi.org/10.1039/c8ta09865d
Roy, S., Cherevotan, A., Peter, S.C.: Thermochemical CO2 hydrogenation to single carbon products: Scientific and technological challenges. ACS Energy Lett. 3, 1938–1966 (2018). https://doi.org/10.1021/acsenergylett.8b00740
Li, Y.H., Li, Q.Y., Wang, H.Q., et al.: Recent progresses in oxygen reduction reaction electrocatalysts for electrochemical energy applications. Electrochem. Energy Rev. 2, 518–538 (2019). https://doi.org/10.1007/s41918-019-00052-4
Global Energy and CO2 Status Report 2018. https://www.iea.org/reports/global-energy-co2-status-report-2019 (2019). Accessed 10 June 2020
Australia wildfires unleash millions of tons of carbon dioxide. https://www.nbcnews.com/science/environment/australia-wildfires-unleash-millions-tons-carbon-dioxide-n1120186 (2020). Accessed 10 June 2020
Antarctic island hits record temperature of 20.75 °C. https://www.bbc.com/news/world-51500692 (2020). Accessed 10 June 2020
Zhang, H.M., Lu, W.J., Li, X.F.: Progress and perspectives of flow battery technologies. Electrochem. Energy Rev. 2, 492–506 (2019). https://doi.org/10.1007/s41918-019-00047-1
Bui, M., Adjiman, C.S., Bardow, A., et al.: Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 11, 1062–1176 (2018). https://doi.org/10.1039/c7ee02342a
Cuéllar-Franca, R.M., Azapagic, A.: Carbon capture, storage and utilisation technologies: a critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 9, 82–102 (2015). https://doi.org/10.1016/j.jcou.2014.12.001
Li, M., Hua, B., Luo, J.L.: Alternative fuel cell technologies for cogenerating electrical power and syngas from greenhouse gases. ACS Energy Lett. 2, 1789–1796 (2017). https://doi.org/10.1021/acsenergylett.7b00392
Zhang, L.X., Hu, S.Q., Li, W.P., et al.: CO2 electroreduction enhanced by transitional layer at cathode/electrolyte interface. J. Power Sources 451, 227743 (2020). https://doi.org/10.1016/j.jpowsour.2020.227743
Wang, W.Y., Gan, L.Z., Lemmon, J.P., et al.: Enhanced carbon dioxide electrolysis at redox manipulated interfaces. Nat. Commun. 10, 1–10 (2019). https://doi.org/10.1038/s41467-019-09568-1
Wu, X.Y., Ghoniem, A.F.: Mixed ionic-electronic conducting (MIEC) membranes for thermochemical reduction of CO2: a review. Prog. Energy Combust. Sci. 74, 1–30 (2019). https://doi.org/10.1016/j.pecs.2019.04.003
Li, J.L., Yoon, H., Wachsman, E.D.: Carbon dioxide reforming of methane in a SrCe0.7Zr0.2Eu0.1O3−δ proton conducting membrane reactor. Int. J. Hydrog. Energy 37, 19125–19132 (2012). https://doi.org/10.1016/j.ijhydene.2012.09.134
Dong, X.L., Jin, W.Q., Xu, N.P., et al.: Dense ceramic catalytic membranes and membrane reactors for energy and environmental applications. Chem. Commun. 47, 10886–10902 (2011). https://doi.org/10.1039/c1cc13001c
Graves, C., Ebbesen, S.D., Mogensen, M., et al.: Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. Renew. Sustain. Energy Rev. 15, 1–23 (2011). https://doi.org/10.1016/j.rser.2010.07.014
Chen, K.F., Jiang, S.P.: Surface segregation in solid oxide cell oxygen electrodes: Phenomena, mitigation strategies and electrochemical properties. Electrochem. Energy Rev. 3, 730–765 (2020). https://doi.org/10.1007/s41918-020-00078-z
Li, X.X., Blinn, K., Chen, D.C., et al.: In situ and surface-enhanced Raman spectroscopy study of electrode materials in solid oxide fuel cells. Electrochem. Energy Rev. 1, 433–459 (2018). https://doi.org/10.1007/s41918-018-0017-9
Zhang, L.X., Hu, S.Q., Zhu, X.F., et al.: Electrochemical reduction of CO2 in solid oxide electrolysis cells. J. Energy Chem. 26, 593–601 (2017). https://doi.org/10.1016/j.jechem.2017.04.004
Song, Y.F., Zhang, X.M., Xie, K., et al.: High-temperature CO2 electrolysis in solid oxide electrolysis cells: developments, challenges, and prospects. Adv. Mater. 31, 1902033 (2019). https://doi.org/10.1002/adma.201902033
Severin, R.F., Izaak, C.V., Lambertus, G.J.H., et al.: Power-to-syngas: an enabling technology for the transition of the energy system? Angew. Chem. Int. Ed. 56, 5402–5411 (2017). https://doi.org/10.1002/anie.201607552
Sapountzi, F.M., Gracia, J.M., Weststrate, C.J.J., et al.: Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 58, 1–35 (2017). https://doi.org/10.1016/j.pecs.2016.09.001
Zheng, Y., Wang, J.C., Yu, B., et al.: A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): advanced materials and technology. Chem. Soc. Rev. 46, 1427–1463 (2017). https://doi.org/10.1039/C6CS00403B
Wang, Y., Liu, T., Lei, L.B., et al.: High temperature solid oxide H2O/CO2 co-electrolysis for syngas production. Fuel Process. Technol. 161, 248–258 (2017). https://doi.org/10.1016/j.fuproc.2016.08.009
Zhang, X.M., Song, Y.F., Wang, G.X., et al.: Co-electrolysis of CO2 and H2O in high-temperature solid oxide electrolysis cells: recent advance in cathodes. J. Energy Chem. 26, 839–853 (2017). https://doi.org/10.1016/j.jechem.2017.07.003
Andika, R., Nandiyanto, A.B.D., Putra, Z.A., et al.: Co-electrolysis for power-to-methanol applications. Renew. Sustain. Energy Rev. 95, 227–241 (2018). https://doi.org/10.1016/j.rser.2018.07.030
Uhm, S., Kim, Y.D.: Electrochemical conversion of carbon dioxide in a solid oxide electrolysis cell. Curr. Appl. Phys. 14, 672–679 (2014). https://doi.org/10.1016/j.cap.2014.02.013
Xu, X.M., Pan, Y.L., Zhong, Y.J., et al.: Ruddlesden-Popper perovskites in electrocatalysis. Mater. Horiz. 7, 2519–2565 (2020). https://doi.org/10.1039/d0mh00477d
Zhu, X.F., Yang, W.S.: Microstructural and interfacial designs of oxygen-permeable membranes for oxygen separation and reaction-separation coupling. Adv. Mater. 31, 1902547 (2019). https://doi.org/10.1002/adma.201902547
Lei, L.B., Zhang, J.H., Yuan, Z.H., et al.: Progress report on proton conducting solid oxide electrolysis cells. Adv. Funct. Mater. 29, 1903805 (2019). https://doi.org/10.1002/adfm.201903805
Medvedev, D.A., Lyagaeva, J.G., Gorbova, E.V., et al.: Advanced materials for SOFC application: strategies for the development of highly conductive and stable solid oxide proton electrolytes. Prog. Mater. Sci. 75, 38–79 (2016). https://doi.org/10.1016/j.pmatsci.2015.08.001
Cheng, H.D., Wang, X.B., Meng, X.X., et al.: Dual-layer BaCe0.8Y0.2O3–δ-Ce0.8Y0.2O2–δ/BaCe0.8Y0.2O3–δ-Ni hollow fiber membranes for H2 separation. J. Membr. Sci. 601, 117801 (2020). https://doi.org/10.1016/j.memsci.2019.117801
Sridhar, K.R., Vaniman, B.T.: Oxygen production on Mars using solid oxide electrolysis. Solid State Ionics 93, 321–328 (1997). https://doi.org/10.1016/S0167-2738(96)00513-9
Sun, Y.F., Wu, Y.Y., Zhang, Y.Q., et al.: A bifunctional solid oxide electrolysis cell for simultaneous CO2 utilization and synthesis gas production. Chem. Commun. 52, 13687–13690 (2016). https://doi.org/10.1039/c6cc03503e
Lu, J.H., Zhu, C.L., Pan, C.C., et al.: Highly efficient electrochemical reforming of CH4/CO2in a solid oxide electrolyser. Sci. Adv. 4, eaar5100 (2018). https://doi.org/10.1126/sciadv.aar5100
Zhu, C.L., Hou, S.S., Hu, X.L., et al.: Electrochemical conversion of methane to ethylene in a solid oxide electrolyzer. Nat. Commun. 10, 1–8 (2019). https://doi.org/10.1038/s41467-019-09083-3
Song, Y.F., Lin, L., Feng, W.C., et al.: Interfacial enhancement by γ-Al2O3 of electrochemical oxidative dehydrogenation of ethane to ethylene in solid oxide electrolysis cells. Angew. Chem. Int. Ed. 58, 16043–16046 (2019). https://doi.org/10.1002/anie.201908388
Ni, M.: Modeling of a solid oxide electrolysis cell for carbon dioxide electrolysis. Chem. Eng. J. 164, 246–254 (2010). https://doi.org/10.1016/j.cej.2010.08.032
Zhang, H.C., Wang, J.Y., Su, S.H., et al.: Electrochemical performance characteristics and optimum design strategies of a solid oxide electrolysis cell system for carbon dioxide reduction. Int. J. Hydrog. Energy 38, 9609–9618 (2013). https://doi.org/10.1016/j.ijhydene.2013.05.155
Shi, Y.X., Luo, Y., Cai, N.S., et al.: Experimental characterization and modeling of the electrochemical reduction of CO2 in solid oxide electrolysis cells. Electrochim. Acta 88, 644–653 (2013). https://doi.org/10.1016/j.electacta.2012.10.107
Zhang, X.R., Ye, L.T., Li, H., et al.: Electrochemical dehydrogenation of ethane to ethylene in a solid oxide electrolyzer. ACS Catal. 10, 3505–3513 (2020). https://doi.org/10.1021/acscatal.9b05409
Itoh, N., Sanchez, M.A., Xu, W.C., et al.: Application of a membrane reactor system to thermal decomposition of CO2. J. Membr. Sci. 77, 245–253 (1993). https://doi.org/10.1016/0376-7388(93)85073-6
Jin, W.Q., Zhang, C., Chang, X.F., et al.: Efficient catalytic decomposition of CO2 to CO and O2 over Pd/mixed-conducting oxide catalyst in an oxygen-permeable membrane reactor. Environ. Sci. Technol. 42, 3064–3068 (2008). https://doi.org/10.1021/es702913f
Zhang, K., Zhang, G.R., Liu, Z.K., et al.: Enhanced stability of membrane reactor for thermal decomposition of CO2 via porous-dense-porous triple-layer composite membrane. J. Membr. Sci. 471, 9–15 (2014). https://doi.org/10.1016/j.memsci.2014.06.060
Jin, W.Q., Zhang, C., Zhang, P., et al.: Thermal decomposition of carbon dioxide coupled with POM in a membrane reactor. AIChE J. 52, 2545–2550 (2006). https://doi.org/10.1002/aic.10850
Zhang, C., Jin, W.Q., Yang, C., et al.: Decomposition of CO2 coupled with POM in a thin tubular oxygen-permeable membrane reactor. Catal. Today 148, 298–302 (2009). https://doi.org/10.1016/j.cattod.2009.08.007
Wu, X.Y., Ghoniem, A.F.: Hydrogen-assisted carbon dioxide thermochemical reduction on La0.9Ca0.1FeO3−δ membranes: A kinetics study. ChemSusChem 11, 483–493 (2018). https://doi.org/10.1002/cssc.201701372
Sakbodin, M., Wu, Y.Q., Oh, S.C., et al.: Hydrogen-permeable tubular membrane reactor: promoting conversion and product selectivity for non-oxidative activation of methane over an Fe©SiO2 catalyst. Angew. Chem. Int. Ed. 55, 16149–16152 (2016). https://doi.org/10.1002/anie.201609991
Sakbodin, M., Schulman, E., Oh, S.C., et al.: Dual utilization of greenhouse gases to produce C2+ hydrocarbons and syngas in a hydrogen-permeable membrane reactor. J. Membr. Sci. 595, 117557 (2020). https://doi.org/10.1016/j.memsci.2019.117557
Ni, M.: An electrochemical model for syngas production by co-electrolysis of H2O and CO2. J. Power Sources 202, 209–216 (2012). https://doi.org/10.1016/j.jpowsour.2011.11.080
Luo, Y., Shi, Y.X., Li, W.Y., et al.: Dynamic electro-thermal modeling of co-electrolysis of steam and carbon dioxide in a tubular solid oxide electrolysis cell. Energy 89, 637–647 (2015). https://doi.org/10.1016/j.energy.2015.05.150
Du, Y.M., Qin, Y.Z., Zhang, G.B., et al.: Modelling of effect of pressure on co-electrolysis of water and carbon dioxide in solid oxide electrolysis cell. Int. J. Hydrog. Energy 44, 3456–3469 (2019). https://doi.org/10.1016/j.ijhydene.2018.12.078
Li, W.Y., Shi, Y.X., Luo, Y., et al.: Elementary reaction modeling of CO2/H2O co-electrolysis cell considering effects of cathode thickness. J. Power Sources 243, 118–130 (2013). https://doi.org/10.1016/j.jpowsour.2013.05.119
Lee, A.C., Mitchell, R.E., Gür, T.M.: Feasibility of hydrogen production in a steam-carbon electrochemical cell. Solid State Ionics 192, 607–610 (2011). https://doi.org/10.1016/j.ssi.2010.05.034
Lei, L.B., Wang, Y., Fang, S.M., et al.: Efficient syngas generation for electricity storage through carbon gasification assisted solid oxide co-electrolysis. Appl. Energy 173, 52–58 (2016). https://doi.org/10.1016/j.apenergy.2016.03.116
Kyriakou, V., Neagu, D., Zafeiropoulos, G., et al.: Symmetrical exsolution of Rh nanoparticles in solid oxide cells for efficient syngas production from greenhouse gases. ACS Catal. 10, 1278–1288 (2020). https://doi.org/10.1021/acscatal.9b04424
Wang, Y., Liu, T., Fang, S.M., et al.: A novel clean and effective syngas production system based on partial oxidation of methane assisted solid oxide co-electrolysis process. J. Power Sour. 277, 261–267 (2015). https://doi.org/10.1016/j.jpowsour.2014.11.092
Wang, Y., Liu, T., Lei, L.B., et al.: Methane assisted solid oxide co-electrolysis process for syngas production. J. Power Sour. 344, 119–127 (2017). https://doi.org/10.1016/j.jpowsour.2017.01.096
Wang, Y.Q., Han, M.F.: Methane partial oxidation-assisted H2O/CO2 co-electrolysis for syngas production in both electrodes. ECS Trans. 78, 3159–3166 (2017). https://doi.org/10.1149/07801.3159ecst
Xu, H.R., Chen, B., Irvine, J., et al.: Modeling of CH4-assisted SOEC for H2O/CO2 co-electrolysis. Int. J. Hydrog. Energy 41, 21839–21849 (2016). https://doi.org/10.1016/j.ijhydene.2016.10.026
Xie, K., Zhang, Y.Q., Meng, G.Y., et al.: Direct synthesis of methane from CO2/H2O in an oxygen-ion conducting solid oxide electrolyser. Energy Environ. Sci. 4, 195–198 (2011). https://doi.org/10.1039/c1ee01035b
Jensen, S.H., Høgh, J.V.T., Barfod, R., Mogensen, M.B. et al.: High temperature electrolysis of steam and carbon dioxide. In: SønderbergPetersen, L., Larsen, H. (eds.) Energy technologies for post Kyoto targets in the medium term. Proceedings, Risø, Denmark., May 19–21, 2003, pp. 204–215 (2003)
Li, W.Y., Wang, H.J., Shi, Y.X., et al.: Performance and methane production characteristics of H2O–CO2 co-electrolysis in solid oxide electrolysis cells. Int. J. Hydrog. Energy 38, 11104–11109 (2013). https://doi.org/10.1016/j.ijhydene.2013.01.008
Chen, L., Chen, F.L., Xia, C.R.: Direct synthesis of methane from CO2–H2O co-electrolysis in tubular solid oxide electrolysis cells. Energy Environ. Sci. 7, 4018–4022 (2014). https://doi.org/10.1039/c4ee02786h
Lei, L.B., Liu, T., Fang, S.M., et al.: The co-electrolysis of CO2–H2O to methane via a novel micro-tubular electrochemical reactor. J. Mater. Chem. A 5, 2904–2910 (2017). https://doi.org/10.1039/c6ta10252b
Luo, Y., Shi, Y.X., Li, W.Y., et al.: Synchronous enhancement of H2O/CO2 co-electrolysis and methanation for efficient one-step power-to-methane. Energy Convers. Manag. 165, 127–136 (2018). https://doi.org/10.1016/j.enconman.2018.03.028
Chen, B., Xu, H.R., Ni, M.: Modelling of SOEC-FT reactor: pressure effects on methanation process. Appl. Energy 185, 814–824 (2017). https://doi.org/10.1016/j.apenergy.2016.10.095
Luo, Y., Shi, Y.X., Chen, Y.B., et al.: Pressurized tubular solid oxide H2 O/CO 2 coelectrolysis cell for direct power-to-methane. AICHE J. 66, e16896 (2020). https://doi.org/10.1002/aic.16896
Luo, Y., Li, W., Shi, Y., et al.: Experimental characterization and theoretical modeling of methane production by H2O/CO2 co-electrolysis in a tubular solid oxide electrolysis cell. J. Electrochem. Soc. 162, F1129–F1134 (2015). https://doi.org/10.1149/2.0171510jes
Chen, B., Xu, H.R., Chen, L., et al.: Modelling of one-step methanation process combining SOECs and Fischer-tropsch-like reactor. J. Electrochem. Soc. 163, F3001–F3008 (2016). https://doi.org/10.1149/2.0011611jes
Fujiwara, N., Kikuchi, R., Takagaki, A., et al.: Investigation of solid oxide electrolysis cell electrodes for methane synthesis. ECS Trans. 78, 3247–3256 (2017). https://doi.org/10.1149/07801.3247ecst
Jensen, S.H., Graves, C., Mogensen, M., et al.: Large-scale electricity storage utilizing reversible solid oxide cells combined with underground storage of CO2 and CH4. Energy Environ. Sci. 8, 2471–2479 (2015). https://doi.org/10.1039/c5ee01485a
Fujiwara, N., Tada, S., Kikuchi, R.: Power-to-gas systems utilizing methanation reaction in solid oxide electrolysis cell cathodes: a model-based study. Sustainable Energy Fuels 4, 2691–2706 (2020). https://doi.org/10.1039/C9SE00835G
Luo, Y., Wu, X.Y., Shi, Y.X., et al.: Exergy analysis of an integrated solid oxide electrolysis cell-methanation reactor for renewable energy storage. Appl. Energy 215, 371–383 (2018). https://doi.org/10.1016/j.apenergy.2018.02.022
Er-Rbib, H., Kezibri, N., Bouallou, C.: Performance assessment of a power-to-gas process based on reversible solid oxide cell. Front. Chem. Sci. Eng. 12, 697–707 (2018). https://doi.org/10.1007/s11705-018-1774-z
Stempien, J.P., Ni, M., Sun, Q., et al.: Thermodynamic analysis of combined solid oxide electrolyzer and Fischer–Tropsch processes. Energy 81, 682–690 (2015). https://doi.org/10.1016/j.energy.2015.01.013
Botta, G., Solimeo, M., Leone, P., et al.: Thermodynamic analysis of coupling a SOEC in Co-electrolysis mode with the dimethyl ether synthesis. Fuel Cells 15, 669–681 (2015). https://doi.org/10.1002/fuce.201500016
Sun, X.F., Chen, M., Jensen, S.H., et al.: Thermodynamic analysis of synthetic hydrocarbon fuel production in pressurized solid oxide electrolysis cells. Int. J. Hydrog. Energy 37, 17101–17110 (2012). https://doi.org/10.1016/j.ijhydene.2012.08.125
Ruiz-Trejo, E., Irvine, J.T.S.: Ceramic proton conducting membranes for the electrochemical production of syngas. Solid State Ionics 216, 36–40 (2012). https://doi.org/10.1016/j.ssi.2012.01.033
Ruiz-Trejo, E., Irvine, J.T.S.: Electrolysis of CO2 in a proton conducting membrane. Solid State Ionics 252, 157–164 (2013). https://doi.org/10.1016/j.ssi.2013.05.021
Wu, G.J., Xie, K., Wu, Y.C., et al.: Electrochemical conversion of H2O/CO2 to fuel in a proton-conducting solid oxide electrolyser. J. Power Sources 232, 187–192 (2013). https://doi.org/10.1016/j.jpowsour.2013.01.039
Danilov, N., Tarutin, A., Lyagaeva, J., et al.: CO2-promoted hydrogen production in a protonic ceramic electrolysis cell. J. Mater. Chem. A 6, 16341–16346 (2018). https://doi.org/10.1039/C8TA90204F
Shi, N., Xie, Y., Huan, D.M., et al.: Controllable CO2 conversion in high performance proton conducting solid oxide electrolysis cells and the possible mechanisms. J. Mater. Chem. A 7, 4855–4864 (2019). https://doi.org/10.1039/c8ta12458b
Bausá, N., Escolástico, S., Serra, J.M.: Direct CO2 conversion to syngas in a BaCe0.2Zr0.7Y0.1O3–δ-based proton-conducting electrolysis cell. J. CO2 Util. 34, 231–238 (2019). https://doi.org/10.1016/j.jcou.2019.05.037
Duan, C.C., Kee, R., Zhu, H.Y., et al.: Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 4, 230–240 (2019). https://doi.org/10.1038/s41560-019-0333-2
Pu, T.T., Tan, W.Y., Shi, H.G., et al.: Steam/CO2 electrolysis in symmetric solid oxide electrolysis cell with Barium cerate-carbonate composite electrolyte. Electrochim. Acta 190, 193–198 (2016). https://doi.org/10.1016/j.electacta.2015.12.220
Liang, W.Y., Cao, Z.W., He, G.H., et al.: Oxygen transport membrane for thermochemical conversion of water and carbon dioxide into synthesis gas. ACS Sustain. Chem. Eng. 5, 8657–8662 (2017). https://doi.org/10.1021/acssuschemeng.7b01305
Yang, L.C., Ge, X.M., Wan, C.X., et al.: Progress and perspectives in converting biogas to transportation fuels. Renew. Sustain. Energy Rev. 40, 1133–1152 (2014). https://doi.org/10.1016/j.rser.2014.08.008
Wei, T., Jia, L.C., Luo, J.L., et al.: CO2 dry reforming of CH4 with Sr and Ni co-doped LaCrO3 perovskite catalysts. Appl. Surf. Sci. 506, 144699 (2020). https://doi.org/10.1016/j.apsusc.2019.144699
Wei, T., Jia, L.C., Zheng, H.Y., et al.: LaMnO3-based perovskite with in situ exsolved Ni nanoparticles: A highly active, performance stable and coking resistant catalyst for CO2 dry reforming of CH4. Appl. Catal. A: Gen. 564, 199–207 (2018). https://doi.org/10.1016/j.apcata.2018.07.031
Kim, T., Moon, S., Hong, S.I.: Internal carbon dioxide reforming by methane over Ni-YSZ-CeO2 catalyst electrode in electrochemical cell. Appl. Catal. A Gen. 224, 111–120 (2002). https://doi.org/10.1016/S0926-860X(01)00735-9
Belyaev, V.D., Galvita, V.V., Sobyanin, V.A.: Effect of anodic current on carbon dioxide reforming of methane on Pt electrode in a cell with solid oxide electrolyte. React. Kinet. Catal. Lett. 63, 341–348 (1998). https://doi.org/10.1007/BF02475409
Moon, D.J., Ryu, J.W.: Electrocatalytic reforming of carbon dioxide by methane in SOFC system. Catal. Today 87, 255–264 (2003). https://doi.org/10.1016/j.cattod.2003.10.017
Ni, M.: Modeling and parametric simulations of solid oxide fuel cells with methane carbon dioxide reforming. Energy Convers. Manag. 70, 116–129 (2013). https://doi.org/10.1016/j.enconman.2013.02.008
Goula, G., Kiousis, V., Nalbandian, L., et al.: Catalytic and electrocatalytic behavior of Ni-based cermet anodes under internal dry reforming of CH4 + CO2 mixtures in SOFCs. Solid State Ionics 177, 2119–2123 (2006). https://doi.org/10.1016/j.ssi.2006.03.040
Shiratori, Y., Sasaki, K.: NiO-ScSZ and Ni0.9Mg0.1O-ScSZ-based anodes under internal dry reforming of simulated biogas mixtures. J. Power Sour. 180, 738–741 (2008). https://doi.org/10.1016/j.jpowsour.2008.03.001
Papadam, T., Goula, G., Yentekakis, I.V.: Long-term operation stability tests of intermediate and high temperature Ni-based anodes’ SOFCs directly fueled with simulated biogas mixtures. Int. J. Hydrog. Energy 37, 16680–16685 (2012). https://doi.org/10.1016/j.ijhydene.2012.02.147
Sameshima, S., Furukawa, N., Hirata, Y., et al.: Cell performance of SOFC using CH4-CO2 mixed gases. Ceram. Int. 40, 6279–6284 (2014)
Mishina, T., Miya, K., Kikuchi, R., et al.: Ni-SDC based cermets for direct dry reforming of methane on SOFC anode. ECS Trans. 78, 1161–1167 (2017). https://doi.org/10.1149/07801.1161ecst
Hua, B., Yan, N., Li, M., et al.: Toward highly efficient in situ dry reforming of H2S contaminated methane in solid oxide fuel cells via incorporating a coke/sulfur resistant bimetallic catalyst layer. J. Mater. Chem. A 4, 9080–9087 (2016). https://doi.org/10.1039/C6TA02809H
Hua, B., Li, M., Sun, Y.F., et al.: Biogas to syngas: flexible on-cell micro-reformer and NiSn bimetallic nanoparticle implanted solid oxide fuel cells for efficient energy conversion. J. Mater. Chem. A 4, 4603–4609 (2016). https://doi.org/10.1039/c6ta00532b
Hua, B., Li, M., Sun, Y.F., et al.: Grafting doped manganite into nickel anode enables efficient and durable energy conversions in biogas solid oxide fuel cells. Appl. Catal. B: Environ. 200, 174–181 (2017). https://doi.org/10.1016/j.apcatb.2016.07.001
Li, M., Hua, B., Zeng, Y.M., et al.: Thermally stable and coking resistant CoMo alloy-based catalysts as fuel electrodes for solid oxide electrochemical cells. J. Mater. Chem. A 6, 15377–15385 (2018). https://doi.org/10.1039/c8ta04749a
Malavasi, L., Fisher, C.A., Islam, M.S.: Oxide-ion and proton conducting electrolyte materials for clean energy applications: structural and mechanistic features. Chem. Soc. Rev. 39, 4370–4387 (2010). https://doi.org/10.1039/b915141a
Medvedev, D.: Trends in research and development of protonic ceramic electrolysis cells. Int. J. Hydrog. Energy 44, 26711–26740 (2019). https://doi.org/10.1016/j.ijhydene.2019.08.130
Hua, B., Yan, N., Li, M., et al.: Anode-engineered protonic ceramic fuel cell with excellent performance and fuel compatibility. Adv. Mater. 28, 8922–8926 (2016). https://doi.org/10.1002/adma.201602103
Hua, B., Yan, N., Li, M., et al.: Novel layered solid oxide fuel cells with multiple-twinned Ni0.8Co0.2 nanoparticles: the key to thermally independent CO2 utilization and power-chemical cogeneration. Energy Environ. Sci. 9, 207–215 (2016). https://doi.org/10.1039/c5ee03017j
Wan, T.T., Zhu, A.K., Guo, Y.M., et al.: Co-generation of electricity and syngas on proton-conducting solid oxide fuel cell with a perovskite layer as a precursor of a highly efficient reforming catalyst. J. Power Sources 348, 9–15 (2017). https://doi.org/10.1016/j.jpowsour.2017.02.074
Chen, B., Xu, H.R., Sun, Q., et al.: Syngas/power cogeneration from proton conducting solid oxide fuel cells assisted by dry methane reforming: a thermal-electrochemical modelling study. Energy Convers. Manag. 167, 37–44 (2018). https://doi.org/10.1016/j.enconman.2018.04.078
Chen, B., Xu, H.R., Zhang, Y., et al.: Combined methane reforming by carbon dioxide and steam in proton conducting solid oxide fuel cells for syngas/power co-generation. Int. J. Hydrog. Energy 44, 15313–15321 (2019). https://doi.org/10.1016/j.ijhydene.2019.02.244
Hirata, Y., Terasawa, Y., Matsunaga, N., et al.: Development of electrochemical cell with layered composite of the Gd-doped ceria/electronic conductor system for generation of H2-CO fuel through oxidation-reduction of CH4-CO2 mixed gases. Ceram. Int. 35, 2023–2028 (2009). https://doi.org/10.1016/j.ceramint.2008.11.001
Matayoshi, S., Hirata, Y., Sameshima, S., et al.: Electrochemical reforming of CH4-CO2 gas using porous Gd-doped ceria electrolyte with Ni and Ru electrodes. J. Ceram. Soc. Japan 117, 1147–1152 (2009). https://doi.org/10.2109/jcersj2.117.1147
Suga, Y.T., Yoshinaga, R., Matsunaga, N., et al.: Electrochemical reforming of CH4−CO2 mixed gas using porous Gd-doped ceria electrolyte with Cu electrode. Ceram. Int. 38, 6713–6721 (2012). https://doi.org/10.1016/j.ceramint.2012.05.061
Qin, Q.Q., Xie, K., Wei, H.S., et al.: Demonstration of efficient electrochemical biogas reforming in a solid oxide electrolyser with titanate cathode. RSC Adv. 4, 38474–38483 (2014). https://doi.org/10.1039/C4RA05587J
Qin, Q.Q., Ruan, C., Ye, L.T., et al.: Efficient syngas production from methane reforming in solid oxide electrolyser with LSCM cathode loaded with Ni–Cu catalysts. J. Solid State Electrochem. 19, 3389–3399 (2015). https://doi.org/10.1007/s10008-015-2966-9
Buyukaksoy, A., Birss, V.I.: Comparison of the electrochemistry of Ni thin film and Ni-YSZ composite anodes fabricated by polymeric precursor deposition. J. Electrochem. Soc. 163, F1350–F1357 (2016). https://doi.org/10.1149/2.0431613jes
Keyvanfar, P., Hanifi, A.R., Sarkar, P., et al.: Enhancing the stability of infiltrated Ni/YSZ anodes. ECS Trans. 68, 1255–1263 (2015). https://doi.org/10.1149/06801.1255ecst
Qi, W.T., Chen, S.G., Wu, Y.C., et al.: A chromium oxide coated nickel/yttria stabilized zirconia electrode with a heterojunction interface for use in electrochemical methane reforming. RSC Adv. 5, 47599–47608 (2015). https://doi.org/10.1039/c5ra01927c
Wei, Y.Y., Huang, L., Tang, J., et al.: Syngas production in a novel perovskite membrane reactor with co-feed of CO2. Chin. Chem. Lett. 22, 1492–1496 (2011). https://doi.org/10.1016/j.cclet.2011.05.040
Yang, N.T., Kathiraser, Y., Kawi, S.: La0.6Sr0.4Co0.8Ni0.2O3−δ hollow fiber membrane reactor: Integrated oxygen separation - CO2 reforming of methane reaction for hydrogen production. Int. J. Hydrog. Energy 38, 4483–4491 (2013). https://doi.org/10.1016/j.ijhydene.2013.01.073
Slade, D.A., Duncan, A.M., Nordheden, K.J., et al.: Mixed-conducting oxygen permeable ceramic membranes for the carbon dioxide reforming of methane. Green Chem. 9, 577–581 (2007). https://doi.org/10.1039/b614232j
Jiang, Q.Y., Faraji, S., Nordheden, K.J., et al.: CO2 reforming reaction assisted with oxygen permeable Ba0.5Sr0.5Co0.8Fe0.2Ox ceramic membranes. J. Membr. Sci. 368, 69–77 (2011). https://doi.org/10.1016/j.memsci.2010.11.011
Kathiraser, Y., Wang, Z.G., Kawi, S.: Oxidative CO2 reforming of methane in La0.6Sr0.4Co0.8Ga0.2O3–δ (LSCG) hollow fiber membrane reactor. Environ. Sci. Technol. 47, 14510–14517 (2013). https://doi.org/10.1021/es403158k
He, G.H., Hu, T.M., Zhou, H.Y., et al.: Syngas production by biogas reforming in a redox-stable and CO2-tolerant oxygen transporting membrane reactor. Ind. Eng. Chem. Res. 56, 10134–10141 (2017). https://doi.org/10.1021/acs.iecr.7b01422
Karagiannakis, G., Zisekas, S., Stoukides, M.: Hydrogenation of carbon dioxide on copper in a H+ conducting membrane-reactor. Solid State Ionics 162(163), 313–318 (2003). https://doi.org/10.1016/S0167-2738(03)00227-3
Xie, K., Zhang, Y.Q., Meng, G.Y., et al.: Electrochemical reduction of CO2 in a proton conducting solid oxide electrolyser. J. Mater. Chem. 21, 195–198 (2011). https://doi.org/10.1039/c0jm02205e
Shin, T.H., Myung, J.H., Naeem, K.M., et al.: Ce(Mn, Fe)O2-(La, Sr)(Fe, Mn)O3 composite as an active cathode for electrochemical reduction of CO2 in proton conducting solid oxide cells. Solid State Ionics 275, 106–109 (2015). https://doi.org/10.1016/j.ssi.2015.03.015
Shang, Y.Y., Wei, L.Y., Meng, X.X., et al.: CO2-enhanced hydrogen permeability of dual-layered A-site deficient Ba0.95Ce0.85Tb0.05Zr0.1O3–δ-based hollow fiber membrane. J. Membr. Sci. 546, 82–89 (2018). https://doi.org/10.1016/j.memsci.2017.10.012
Wei, S.S., Wang, T.H., Wu, J.S.: Numerical modeling of interconnect flow channel design and thermal stress analysis of a planar anode-supported solid oxide fuel cell stack. Energy 69, 553–561 (2014). https://doi.org/10.1016/j.energy.2014.03.052
Dillig, M., Plankenbühler, T., Karl, J.: Thermal effects of planar high temperature heat pipes in solid oxide cell stacks operated with internal methane reforming. J. Power Sour. 373, 139–149 (2018). https://doi.org/10.1016/j.jpowsour.2017.11.007
Takamura, H., Ogawa, M., Suehiro, K., et al.: Fabrication and characteristics of planar-type methane reformer using ceria-based oxygen permeable membrane. Solid State Ionics 179, 1354–1359 (2008). https://doi.org/10.1016/j.ssi.2008.04.001
Kim, S.D., Yu, J.H., Seo, D.W., et al.: Hydrogen production performance of 3-cell flat-tubular solid oxide electrolysis stack. Int. J. Hydrog. Energy 37, 78–83 (2012). https://doi.org/10.1016/j.ijhydene.2011.09.079
Rashid, K., Dong, S.K., Mehran, M.T.: Numerical investigations to determine the optimal operating conditions for 1 kW-class flat-tubular solid oxide fuel cell stack. Energy 141, 673–691 (2017). https://doi.org/10.1016/j.energy.2017.09.082
Park, B.K., Lee, J.W., Lee, S.B., et al.: A flat-tubular solid oxide fuel cell with a dense interconnect film coated on the porous anode support. J. Power Sour. 213, 218–222 (2012). https://doi.org/10.1016/j.jpowsour.2012.04.025
Kim, D.W., Yun, U.J., Lee, J.W., et al.: Fabrication and operating characteristics of a flat tubular segmented-in-series solid oxide fuel cell unit bundle. Energy 72, 215–221 (2014). https://doi.org/10.1016/j.energy.2014.05.026
Lee, D.Y., Mehran, M.T., Kim, J., et al.: Scaling up syngas production with controllable H2/CO ratio in a highly efficient, compact, and durable solid oxide coelectrolysis cell unit-bundle. Appl. Energy 257, 114036 (2020). https://doi.org/10.1016/j.apenergy.2019.114036
Wang, H.H., Feldhoff, A., Caro, J., et al.: Oxygen selective ceramic hollow fiber membranes for partial oxidation of methane. AICHE J. 55, 2657–2664 (2009). https://doi.org/10.1002/aic.11856
Izuki, M., Brito, M.E., Yamaji, K., et al.: Interfacial stability and cation diffusion across the LSCF/GDC interface. J. Power Sour. 196, 7232–7236 (2011)
Pan, Z.H., Liu, Q.L., Lyu, R.Z., et al.: Effect of La0.6Sr0.4Co0.2Fe0.8O3–δ air electrode-electrolyte interface on the short-term stability under high-current electrolysis in solid oxide electrolyzer cells. J. Power Sour. 378, 571–578 (2018). https://doi.org/10.1016/j.jpowsour.2018.01.002
Tsoga, A., Gupta, A., Naoumidis, A., et al.: Gadolinia-doped ceria and yttria stabilized zirconia interfaces: regarding their application for SOFC technology. Acta Mater. 48, 4709–4714 (2000). https://doi.org/10.1016/S1359-6454(00)00261-5
Fu, C.J., Liu, Q.L., Chan, S.H., et al.: Effects of transition metal oxides on the densification of thin-film GDC electrolyte and on the performance of intermediate-temperature SOFC. Int. J. Hydrog. Energy 35, 11200–11207 (2010). https://doi.org/10.1016/j.ijhydene.2010.07.049
Nicollet, C., Waxin, J., Dupeyron, T., et al.: Gadolinium doped ceria interlayers for solid oxide fuel cells cathodes: enhanced reactivity with sintering aids (Li, Cu, Zn), and improved densification by infiltration. J. Power Sources 372, 157–165 (2017). https://doi.org/10.1016/j.jpowsour.2017.10.064
Lee, S., Lee, S., Kim, H.J., et al.: Highly durable solid oxide fuel cells: Suppressing chemical degradation viarational design of a diffusion-blocking layer. J. Mater. Chem. A 6, 15083–15094 (2018). https://doi.org/10.1039/c8ta04974b
Tosti, S., Borgognoni, F., Rizzello, C., et al.: Water gas shift reaction via Pd-based membranes. Asia-Pacific JRNL Chem. Eng 4, 369–379 (2009). https://doi.org/10.1002/apj.253
Yan, X.H., Guan, C., Zhang, Y., et al.: Flow field design with 3D geometry for proton exchange membrane fuel cells. Appl. Therm. Eng. 147, 1107–1114 (2019). https://doi.org/10.1016/j.applthermaleng.2018.09.110
Liu, H.C., Yang, W.M., Tan, J., et al.: Numerical analysis of parallel flow fields improved by micro-distributor in proton exchange membrane fuel cells. Energy Convers. Manag. 176, 99–109 (2018). https://doi.org/10.1016/j.enconman.2018.09.024
Wang, Y., Du, Y.M., Ni, M., et al.: Three-dimensional modeling of flow field optimization for co-electrolysis solid oxide electrolysis cell. Appl. Therm. Eng. 172, 114959 (2020). https://doi.org/10.1016/j.applthermaleng.2020.114959
Ding, D., Li, X.X., Lai, S.Y., et al.: Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 7, 552–575 (2014). https://doi.org/10.1039/C3EE42926A
Xu, X.M., Wang, W., Zhou, W., et al.: Recent advances in novel nanostructuring methods of perovskite electrocatalysts for energy-related applications. Small Methods 2, 1800071 (2018). https://doi.org/10.1002/smtd.201800071
Zhang, J.W., Gao, M.R., Luo, J.L.: In situ exsolved metal nanoparticles: a smart approach for optimization of catalysts. Chem. Mater. 32, 5424–5441 (2020). https://doi.org/10.1021/acs.chemmater.0c00721
Hua, B., Li, M., Chi, B., et al.: Enhanced electrochemical performance and carbon deposition resistance of Ni–YSZ anode of solid oxide fuel cells by in situ formed Ni–MnO layer for CH4 on-cell reforming. J. Mater. Chem. A 2, 1150–1158 (2014). https://doi.org/10.1039/c3ta12766d
Hua, B., Li, M., Zhang, W.Y., et al.: Methane on-cell reforming by alloys reduced from Ni0.5Cu0.5Fe2O4 for direct-hydrocarbon solid oxide fuel cells. J. Electrochem. Soc. 161, F569–F575 (2014). https://doi.org/10.1149/2.097404jes
Zhang, Y., Knibbe, R., Sunarso, J., et al.: Recent progress on advanced materials for solid-oxide fuel cells operating below 500 ℃. Adv. Mater. 29, 1700132 (2017). https://doi.org/10.1002/adma.201700132
Liu, Y., Zhu, X.F., Li, M.R., et al.: Stabilization of low-temperature degradation in mixed ionic and electronic conducting perovskite oxygen permeation membranes. Angew. Chem. Int. Ed. 52, 3232–3236 (2013). https://doi.org/10.1002/anie.201209077
Yang, G.M., Su, C., Shi, H.G., et al.: Toward reducing the operation temperature of solid oxide fuel cells: our past 15 years of efforts in cathode development. Energy Fuels 34, 15169–15194 (2020). https://doi.org/10.1021/acs.energyfuels.0c01887
Ni, M., Shao, Z.P.: Fuel cells that operate at 300 °C to 500 °C. Science 369, 138–139 (2020). https://doi.org/10.1126/science.abc9136
Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council of Canada, the Discovery Grant (GRPIN-2016-05494) and the Alberta Innovates Technology Futures Research Grant. As a part of the University of Alberta’s Future Energy Systems research initiative, this work was also made possible in part thanks to funding from the Canada First Research Excellence Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Luo, JL. High-Temperature Electrochemical Devices Based on Dense Ceramic Membranes for CO2 Conversion and Utilization. Electrochem. Energ. Rev. 4, 518–544 (2021). https://doi.org/10.1007/s41918-021-00099-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41918-021-00099-2