Abstract
Background and Objective
The phase III ALFA-0701 study demonstrated the efficacy and safety of gemtuzumab ozogamicin (GO) versus standard of care (SOC) chemotherapy (daunorubicin and cytarabine) for the treatment of adult patients with de novo CD33+ acute myeloid leukaemia (AML). This study analysed the cost-effectiveness of GO from the perspective of the UK health care payer.
Methods
A cohort state-transition model was developed to estimate direct health care costs and quality-adjusted life-years (QALYs) over a lifetime time horizon from AML diagnosis to death using monthly cycles. Data on complete remission, overall survival, relapse-free survival (RFS), haematopoietic stem-cell transplantation, and adverse events for GO plus SOC versus SOC were obtained from the ALFA-0701 study. Overall survival and RFS were extrapolated beyond the trial horizon using mixture cure models. Unit costs were obtained from standard national sources. Utilities were identified in a systematic literature review. Costs and outcomes were discounted at 3.5%. Analyses were performed for the base-case population, excluding patients with an unfavourable cytogenetic profile, and the overall population.
Results
For the base-case and overall populations respectively, incremental per-patient costs (£13,456 and £14,773) and QALYs (0.99 and 0.68) for GO plus SOC versus SOC resulted in incremental cost-effectiveness ratios (ICERs) of £13,561 and £21,819 per QALY gained. The mean probabilistic ICERs were £14,217 and £23,245, respectively. Univariate sensitivity analyses supported the robustness of the results.
Conclusions
The ICERs for both populations met NICE’s £20,000–£30,000 willingness-to-pay threshold for medicines and supported the current approval for GO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gemtuzumab ozogamicin (GO), in combination with standard of care (SOC) chemotherapy (daunorubicin plus cytarabine), was approved for the treatment of newly diagnosed patients with CD33+ acute myeloid leukaemia (AML) in 2017 by the US Food and Drug Administration and in 2018 by the European Medicines Agency. |
Results of our cost-effectiveness analysis indicate that GO in combination with SOC is a cost-effective first-line treatment option for adult patients with de novo AML from the perspective of the UK health care payer. |
The analyses presented in this article supported the submission leading to the UK National Institute for Health and Care Excellence recommendation for GO plus SOC as a treatment option for patients with untreated CD33+ AML with favourable, intermediate, or unknown cytogenetics. |
1 Introduction
Acute myeloid leukaemia (AML) is the most common type of acute leukaemia in adults [1]. In the United Kingdom (UK), the incidence of AML is ~ 3100 new cases every year; incidence rates have increased by 29% since the early 1990s [2]. The total estimated prevalence of AML in the UK is 9.6/100,000, based on the number of newly diagnosed cases in 2004–2011 and patient survival [3].
Acute myeloid leukaemia is associated with a short life expectancy and is a terminal condition if left untreated. Approximately 20% of patients with AML will survive for ≥ 5 years after diagnosis [2]. Although most adult patients can achieve complete remission (CR) following standard induction chemotherapy, many patients will eventually relapse [4, 5]. There are ~ 2600 annual deaths from AML in the UK [2].
Chromosomal abnormalities, as detected by cytogenetics profile, are the most powerful prognostic factor for predicting the response to treatment and the risk of relapse [4, 6]. Based on diagnostic karyotyping, patients can be characterised as having a favourable, intermediate, or unfavourable cytogenetics profile according to the types of abnormalities that are present [6]. Those who have not received cytogenetic test results are classified as having unknown cytogenetics. Cytogenetic abnormalities have been identified in approximately half of all patients with newly diagnosed AML and the incidence of unfavourable cytogenetic abnormalities increases with age [6]. Patients with a favourable or intermediate cytogenetics profile have a better prognosis than those with an unfavourable cytogenetics profile [7,8,9], which can inform treatment strategies. After patients attain CR, physicians should decide early on whether haematopoietic stem-cell transplantation (HSCT) is needed for those at high risk of relapse who cannot maintain CR with chemotherapy [4,5,6,7,8,9,10].
The treatment landscape for AML is rapidly changing, with the approval of several novel therapies beginning in 2017 [11]. Gemtuzumab ozogamicin (GO; Mylotarg™) is a CD33-directed antibody conjugated to a potent, cytotoxic calicheamicin derivative. The CD33 antigen is expressed on at least a subset of AML cells in almost all patients and represents an important target for antibody-based AML therapy [12]. Gemtuzumab ozogamicin, in combination with standard-of-care (SOC) chemotherapy (daunorubicin + cytarabine), was approved for the treatment of newly diagnosed patients with CD33+ AML in 2017 by the US Food and Drug Administration and in 2018 by the European Medicines Agency [13, 14]. The approval of GO + SOC was based, in part, on the results from the phase III ALFA-0701 study (ClinicalTrials.gov identifier NCT00927498), which demonstrated significantly longer event-free survival with a fractionated dose of GO + SOC versus SOC alone [17]. The benefit of GO was particularly evident in the subgroup of patients with favourable or intermediate cytogenetics, whereas the addition of GO did not have a significant impact on patients with unfavourable cytogenetics.
The present study assessed the cost effectiveness of GO + SOC versus SOC alone in the first-line treatment of adult patients with de novo CD33+ AML. The analysis was performed from the perspective of the UK health care payer.
2 Methods
2.1 Model Overview
A cohort state-transition model was developed in Microsoft Excel (Microsoft Corporation) to evaluate the costs and effectiveness of GO + SOC versus SOC alone for de novo CD33+ AML patients, from treatment initiation to death. Analyses were performed from the perspective of the UK health care payer, which included all direct health care costs (National Health Service [NHS] and Personal Social Services) with a cost year of 2017. Indirect and non-health care costs were not included. The effectiveness measures included quality-adjusted life-years (QALYs) and life-years (LYs). Costs and health outcomes were discounted at 3.5% per annum [16]. A lifetime time horizon was used; the analysis time frame was 40 years, with a cycle length of 1 month. A half-cycle correction was applied.
The modelled population consisted of adult patients with previously untreated de novo CD33+ AML who were eligible to receive intensive chemotherapy. Separate analyses were performed for two patient populations.
-
Base-case population: the subgroup that had a clear benefit with the addition of GO and excluded patients with unfavourable cytogenetics (i.e., favourable, intermediate, or unknown cytogenetics).
-
Overall population: all patients, regardless of favourable, intermediate, unfavourable, or unknown cytogenetic profile.
The treatment regimens for the model comparators aligned with the ALFA-0701 study [17], which informed the licence for GO (Table 1). In the ALFA-0701 study, patients were randomised to receive standard first-line induction chemotherapy (3+7 daunorubicin + cytarabine) plus GO 3 mg/m2 (capped at 5 mg) on Days 1, 4, and 7 during induction (GO + SOC arm) or SOC alone (SOC arm). Patients in CR following induction therapy received up to two courses of consolidation therapy (daunorubicin + cytarabine) alone or with GO 3 mg/m2 (capped at 5 mg) on Day 1, according to their initial randomisation.
2.2 Model Structure
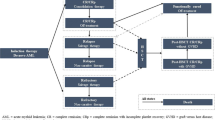
The model structure diagram is presented in Fig. 1. De novo AML patients enter the model on commencement of their systemic therapy (either GO + SOC or SOC alone). All patients received one induction course. A second induction course of SOC only (without GO) was possible for all patients without an adequate response to the first induction course. At the end of induction therapy, patients were assessed and either attained CR or CR with incomplete platelet recovery (CRp) (induction success) or were refractory to induction therapy (induction failure). All patients left the induction therapy health state after two cycles. Patients who attained CR or CRp continued with up to two courses of consolidation therapy then moved off treatment. Patients with certain risk profiles who attained CR or CRp received HSCT instead of consolidation therapy if it was considered beneficial. The proportion of patients in the ALFA-0701 study who received each course of induction and consolidation treatment was applied in the model to account for treatment discontinuation (Table 1).
Model structure diagram. Induction therapy captures the initial period of treatment with GO + SOC or SOC alone prior to determination of response status. CR or CRp and refractory health states capture treatment phases for patients with induction success and failure, respectively. Relapse health states capture treatment phases for patients with disease progression following CR or CRp. HSCT captures the period of HSCT procedure and recovery when patients remain hospitalised. Post-HSCT CR/CRp health states capture the period after HSCT procedure prior to becoming ‘functionally cured’. Functionally cured captures long-term disease-free survival (CR or CRp) with no planned follow-up. AML acute myeloid leukaemia, CR complete remission, CRp CR with incomplete platelet recovery, GVHD graft-versus-host disease, HSCT haematopoietic stem-cell transplantation
A proportion of patients entering the relapse or refractory health states who were deemed sufficiently fit to receive high-intensity chemotherapy (based on clinical input) began up to two courses of salvage therapy (consisting of fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin [FLAG-Ida]) with the aim to attain second-line CR or CRp and transplant (Table 1). The remaining proportion of patients who were not deemed sufficiently fit to receive high-intensity chemotherapy began non-curative therapies (azacitidine, low-dose cytarabine, or hydroxycarbamide [best supportive care]), followed by palliative care. Patients who transitioned to the HSCT health state from the CR or CRp, relapse and refractory health states remained there for one model cycle to account for the HSCT procedure. Patients who received HSCT then moved to the post-HSCT CR or CRp health state, with or without graft-versus-host disease (GVHD).
Patients who had CR or CRp after 5 years (in the CR/CRp or post-HSCT CR/CRp health states) transitioned to the functionally cured health state. Clinical advisers in the UK considered 5 years to be a robust estimate for functional cure (long-term disease-free survival) for all alive patients.
2.3 Model Inputs
A summary of the variables applied in the economic model is presented in Tables 2 and 3.
2.3.1 Event Probabilities
The modified intention-to-treat (mITT) population of the ALFA-0701 study [17] was used to inform transition probabilities and events. Response rates and adverse event (AE) probabilities (Table 2) were taken directly from the ALFA-0701 study, whereas parametric survival functions for relapse-free survival (RFS) and overall survival (OS) and HSCT probabilities were derived from patient-level data for the mITT population. The overall mITT population included 271 patients: 135 in the GO + SOC arm and 136 in the SOC arm. The base-case mITT population (excluding patients with unfavourable cytogenetics) included 108 and 106 patients, respectively. Response was measured in terms of CR or CRp at the end of induction therapy. Patients who did not attain CR or CRp were categorised as failing induction therapy.
Parametric survival functions were fitted to RFS and OS. Overall survival was stratified by response status because survival for patients who attained CR or CRp was expected to be substantially longer than that for refractory patients. Moreover, GO is known to extend RFS [17]; therefore, to account for this added benefit and generate a meaningful comparison, OS curves were divided according to response. Validation was performed to ensure that the stratified OS curves summed to the overall OS curve. Clinical advisers believed that GO would not affect OS for refractory patients since patients failing induction treatment tend to demonstrate poor prognosis and survival outcomes after treatment failure. Therefore, OS for refractory patients was pooled in the base-case analysis.
To capture cure rates associated with AML, we explored parametric and more complex models (flexible-spline and mixture cure models [MCMs]). Mixture cure models are well established statistical practice for studies in AML disease [18,19,20,21,22,23,24,25]; MCMs were fitted by using the strsmix package in STATA (StataCorp LLC) and provided the best statistical fit and most plausible survival projections for RFS and OS (CR or CRp).
A hazard ratio (base-case population = 1.36) for excess mortality versus the general population was calculated using an analysis of pooled survival data from UK AML trials 10–16. The hazard ratio was applied after the ALFA-0701 study follow-up period (5 years) when patients were considered functionally cured. The survival extrapolations used in the base-case analysis are presented in Fig. 2 for the base-case population and in Online Resource 1, eFigure 1 (see electronic supplementary material [ESM]), for the overall population.
Patients could receive HSCT from the CR or CRp, relapse, and refractory health states. Separate HSCT probabilities were calculated for each of these health states (Table 2). The probabilities were calculated from the number of patients who underwent HSCT using ALFA-0701 study time-to-HSCT analyses for the total cohorts of patients who attained CR or CRp (without relapse), who relapsed, and who were refractory to induction therapy.
2.3.2 Health Utility Estimates
Health-related quality-of-life data were not collected during the ALFA-0701 study. Health utility estimates were identified by a systematic literature review and a preference elicitation study in which preference values were assigned to health states experienced by AML patients, as described in vignettes, from the perspective of the general UK population [15].
The health-state utility values included in the base case are presented in Table 2. Health-state utility estimates using the EQ-5D were chosen for the base-case analysis because this is the preferred measure for the UK [16]. For the functionally cured health state, age-adjusted EQ-5D values were used for the UK general population and calculated from the formula reported by Ara and Brazier [26].
Utility decrements for AEs were applied in the model to capture treatment-specific utility loss due to toxicity. A mean utility decrement of 0.0207 (National Institute for Health and Care Excellence [NICE] Technology Appraisal No. 399; Pfizer data on file, 2015) was applied as a one-time decrement for all grade 3 and 4 AEs except for veno-occlusive disease (VOD) (Table 3). A utility decrement of 0.208 [27] was applied for VOD for a mean duration of 26.8 days [28].
2.3.3 Resource Use and Costs
A systematic literature review was conducted to identify primary studies reporting health care resource use and costs. Costs estimated by the model included first- and subsequent-line treatment costs, HSCT costs, the costs of treating AEs, health-state costs (including hospitalisations, specialist consultations, diagnostics, supportive therapies, and blood transfusions), and terminal care costs.
Unit costs were taken from recognised national sources (where available), including the British National Formulary (BNF) [29], drugs and pharmaceutical electronic market information [30], NHS Reference Costs [31], and the published literature. Costs quoted for other cost-years or in other currencies were inflated to 2017 costs or converted to the currency of the country of analysis, as applicable. Resource utilisation estimates were taken from the ALFA-0701 study and the published literature or based on clinical opinion.
2.4 Sensitivity Analysis
Univariate sensitivity analysis was performed to identify the parameters that had the most influence on the incremental cost-effectiveness ratio (ICER). Scenario analyses exploring structural uncertainty for specifically identified areas of uncertainty were performed.
Probabilistic sensitivity analysis was conducted to evaluate uncertainty associated with parameter precision. Probabilistic sensitivity analyses included all model parameters; estimates of uncertainty were based on the uncertainty in the source data (where data availability permitted this). In cases where this was permitted, exact data were used to capture the upper and lower bounds; in instances of a lack of data, 10% variability from mean values was applied. All parameters were varied simultaneously, and multiple sets of parameter values were sampled from predefined probability distributions in order to characterise the uncertainty associated with the precision of mean parameter values.
2.5 Model Validation
The model incorporated information from relevant literature, previous health technology assessment appraisals, and constructive feedback on model design from 11 clinical, statistical, and health economics experts (acknowledged in Declarations). Model validation was performed in alignment with best practice [32].
The model specifications (including the model structure, key data sources, and assumptions) were reviewed by clinical experts and external health economists to align with NICE Decision Support Unit (DSU) No. 14 [33] best practice methods for validation of long-term extrapolated outcomes. The statistical analysis plans for survival analysis were reviewed by an external statistician.
Quality control procedures for verification of input data and coding were performed by RTI Health Solutions staff not involved in the model development and in accordance with a prespecified test plan (procedures included verification of all input data with original sources and programming validation).
The model also was validated by an external health economics consultant who was asked to evaluate the model from the perspective of NICE’s Evidence Review Group. Model predictions were validated against the ALFA-0701 study data and external data (long-term pooled analysis of UK AML trials).
3 Results
In the base case (subgroup of patients with favourable, intermediate, or unknown cytogenetics), the deterministic analysis found higher per-patient costs (£135,545 vs £122,088), greater number of LYs (7.24 vs 5.93), and greater number of QALYs (5.29 vs 4.30) for GO + SOC versus SOC (Table 4). Corresponding ICERs were £10,240/LY and £13,561/QALY gained. The mean probabilistic ICER was £14,217/QALY gained (95% confidence interval [CI] 12,985–15,587) (Fig. 3), with a 77% probability of being cost effective at a willingness-to-pay threshold of £30,000/QALY (Fig. 4).
In the overall population (now also including those with unfavourable cytogenetics), there were again higher per-patient costs (£132,245 vs £117,472), greater LYs (6.17 vs 5.28) and greater QALYs (4.51 vs 3.83) associated with GO + SOC versus SOC (Table 4). Corresponding ICERs were £16,492/LY and £21,819/QALY gained. The mean probabilistic ICER was £23,245/QALY gained (95% CI 20,911–26,039) (Fig. 3), with a 60% probability of being cost effective at a willingness-to-pay threshold of £30,000/QALY (Fig. 4).
Higher costs of GO + SOC were mainly attributable to drug acquisition; however, these were partially offset via cost savings seen from relapse prevention and fewer HSCTs. In the base-case population, the parameters that had the largest impact on the ICER when they were increased or decreased in the univariate sensitivity analysis were generally those related to HSCT (Online Resource 1, eFigure 2, see ESM). The ICER was insensitive to changes in individual parameters; HSCT probabilities from the relapse health state in years 1 and 2 were most impactful but changed the ICER by < £1000/QALY. Similar results were observed in the overall population.
Scenario analysis results are presented in Table 5. For the base-case population, the ICER was most sensitive to assumptions about the pooling of response rates and the OS curve for refractory patients. Pooled data from ALFA-0701 were used in the model based on clinical opinion that no differences are expected between treatment arms. Using individual treatment-arm data for response rates decreased the ICER by £3035 and using individual OS (refractory) curves increased the ICER by £3714. For the overall population, the ICER decreased by £5219 using response rates for individual arms and increased by £1267 using individual OS (refractory) curves. The ICER was also sensitive to the choice of health-state utility values. Using time trade-off values from the preference elicitation study [15] that were considerably lower than the EQ-5D values increased the base-case population ICER by £1578 and increased the overall population ICER by £4729.
4 Discussion
An economic model was developed to assess the cost effectiveness of GO + SOC versus SOC alone in the first-line treatment of adult patients with de novo CD33+ AML from the perspective of the UK health care payer. Our analyses support the UK NICE recommendation of GO + SOC as a treatment option for patients with untreated CD33+ AML with favourable, intermediate, or unknown cytogenetics [34]. The NICE recommendation includes patients whose cytogenetics are unknown because the test is unsuccessful or because test results are not yet available. These patients discontinue GO if test results show them to have unfavourable cytogenetics.
The base-case population analysis presented in this article represents the base-case analysis submitted to NICE without the confidential patient access scheme discount for GO. Minor errors identified by the Evidence Review Group [34] have been corrected, but the assumptions proposed by the Evidence Review Group for their alternative base-case analysis have not been used. NICE reported that their preferred analysis, representing the most plausible ICER for the base-case population, was below £20,000/QALY gained [34].
NICE included a stopping rule for patients with unknown cytogenetics whose test results show them to have unfavourable cytogenetics. Cost offsets were applied for the following patients who would stop GO:
-
Patients waiting for test results who do not need urgent treatment were assumed not to receive GO.
-
Patients waiting for test results who do need urgent treatment were assumed to receive GO in the first induction cycle but stop before receiving consolidation therapy with GO.
In total, a stopping rule was applied to 0.7% of the base-case population. The stopping rule was replicated in a scenario analysis for the base-case population presented in this article, which resulted in a small reduction to the ICER of £163 (Table 5).
Midostaurin in combination with SOC was recommended by NICE in 2018 as an option for treating adults with newly diagnosed FLT3-mutation-positive AML [45]. Midostaurin was not identified as a comparator to GO during the NICE appraisal process, and no comparison was attempted. Only 16% of patients who received GO in the ALFA-0701 trial were reported as FLT3-mutation positive in the trial’s primary publication [17], which makes a meaningful comparison difficult.
4.1 Study Strengths
A transparent, probabilistic, cost-effectiveness model was developed in Microsoft Excel and Microsoft Visual Basic for Applications. The model was developed to meet the standards required by NICE [16]. A semi-Markov cohort state-transition model with 12 health states was used to capture differences in costs and outcomes throughout the entire disease course and patient lifetime. Relevant health states were identified and validated as part of a preference elicitation study [15] and were considered to comprehensively reflect the experience of AML patients throughout the treatment pathway. The model structure is more complex than a simpler partitioned survival model, as was used for midostaurin [45], and does not directly use the underlying efficacy data, which could add uncertainty. However, moving from a partitioned survival model to a state-transition model allowed additional health states to be modelled. Overall survival was stratified by response status to isolate the benefit of GO for patients who achieve CR/CRp and generate a meaningful comparison. Validation was performed to ensure that the stratified OS curves summed to the overall OS curve.
The transitions between the main health states were governed by the parametric functions fitted to patient-level RFS and OS data in the ALFA-0701 study. Advanced modelling techniques were used to ensure that the tail of the data, representing the proportion of cured patients, was modelled appropriately to enable the accurate projection of long-term outcomes. Additional transitions were included to capture second-line treatments and HSCTs based on analyses of patient-level data in the ALFA-0701 study. Clinical assumptions and survival extrapolations were cross-validated with external data and UK clinical experts to ensure the assumptions and extrapolations aligned with expectations in clinical practice. A cure fraction parameter was estimated for different MCM functions and varied in the probabilistic sensitivity analysis to reflect stochastic uncertainty. The difference in the cure fraction between treatment groups, which is the main driver of incremental QALYs, was similar across different MCM functions for OS and RFS.
The NICE appraisal committee concluded that the ALFA-0701 study population was generalisable to the population who would be eligible for GO + SOC in clinical practice in England [34]. Moreover, the application of GO in the model aligned with the European licence [14] and was costed using the UK list price. The model provided results relevant to the UK payer perspective that were applicable for de novo CD33+ AML seen in UK clinical practice.
Extensive sensitivity analysis was performed, including univariate and probabilistic sensitivity analyses incorporating all model parameters, and scenario analyses exploring structural uncertainty (e.g., alternative survival functions) for specifically identified areas of uncertainty (e.g., alternative utility weights). The sensitivity analyses demonstrated the robustness of the model results.
4.2 Study Limitations
No previous peer-reviewed articles containing economic analyses were identified for GO in de novo AML; thus, it was not possible to compare the model results with published literature.
The model structure was considered appropriate for decision making by the NICE appraisal committee [34] but was complex and relied on clinical opinion where data were not available. The time until patients with long-term disease-free survival are classified as being functionally cured was estimated by clinical experts to be 3–5 years. The time at which patients transition to the functionally cured health state in the model was 5 years and this was considered a conservative estimate; timepoints < 5 years resulted in lower ICERs. The proportion of relapsed and refractory patients who received salvage therapy was estimated to be 60% by clinical experts. The ICER was very insensitive to changes in this value because pooled response rates were used, meaning that the proportion of patients who were refractory and who attained CR/CRp and could relapse was the same for GO + SOC and SOC. The duration of post-HSCT GVHD was also estimated by clinicians and had a minimal impact on the ICER when varied in the univariate sensitivity analysis.
Health-related quality-of-life data were not collected in the ALFA-0701 study; therefore, the utility estimates were obtained from other sources. There was a paucity of available data in AML, and estimates were taken from different patient populations. Appropriate EQ-5D utility estimates were not identified for all model health states. The utility value for the refractory health state was assumed to be equal to the relapse health state. The utilities for patients receiving consolidation chemotherapy and undergoing an HSCT procedure were assumed to equal the utility for patients receiving high-intensity chemotherapy. These assumptions were validated by UK clinicians.
The majority of the QALY gains in the model were generated in the functionally cured and CR/CRp (off-treatment) health states; consequently, the ICER was most sensitive to utility values for these health states. Age-adjusted utility values for the UK general population were calculated from the formula reported by Ara and Brazier [26] for the functionally cured health state and were considered the least uncertain utility values. The utility value for the CR/CRp (off-treatment) health state was taken from NICE Technology Appraisal 399 (Pfizer data on file, 2015); the value was mapped to the EQ-5D from trial-based, disease-specific EORTC QLQ-C30 data using a published algorithm [44]. Applying lower utility values for these health states would increase the ICER. This was demonstrated in a scenario analysis using alternative health-state utility estimates obtained from a preference elicitation study [15] in which the ICERs for the base case and overall populations increased by £1578 and £4729, respectively (Table 5).
5 Conclusions
Gemtuzumab ozogamicin in combination with SOC is a cost-effective first-line treatment option for adult patients with de novo AML. The increased costs of adding GO to SOC (daunorubicin and cytarabine) were partially offset by improved clinical outcomes compared with SOC alone. At the UK list price for GO, the ICERs for the base-case and overall populations meet the UK’s £20,000–£30,000 willingness-to-pay threshold for medicines [16]. The results were more beneficial for the base-case population, excluding those with unfavourable cytogenetics, than the overall population. NICE’s preferred analysis for the base-case population produced similar results, with an ICER below £20,000/QALY gained, and led to their recommendation for GO + SOC as a treatment option for patients with untreated CD33+ AML [34].
References
National Cancer Institute (NCI). SEER cancer stat facts: acute myeloid leukemia. https://seer.cancer.gov/statfacts/html/amyl.html. Accessed 28 Nov 2019.
Cancer Research UK. Acute myeloid leukaemia (AML) statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/leukaemia-aml#heading-Zero. Accessed 28 Nov 2019.
Li J, Smith A, Crouch S, Roman E. Estimating the prevalence of hematological malignancies and precursor conditions using data from Haematological Malignancy Research Network (HMRN). Cancer Causes Control. 2016;27:1019–26.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127:53–61.
Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer. 2011;2:95–107.
De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441.
Liersch R, Muller-Tidow C, Berdel WE, Krug U. Prognostic factors for acute myeloid leukaemia in adults—biological significance and clinical use. Br J Haematol. 2014;165:17–38.
Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100:4325–36.
EBMT-ESH handbook on haematopoietic stem cell transplantation (2012 revised edition). In: Apperley J, Carreras E, Gluckman E, Masszi T, editors. Chapter 19.1 indictions for HSCT in adults: acute myeloid leukaemia. https://ebmtonline.forumservice.net/media/19_1/tex/content_alt/EBMT_Handbook2012_CHAP19_1.pdf. Accessed 14 Aug 2017.
Talati C, Sweet K. Recently approved therapies in acute myeloid leukemia: a complex treatment landscape. Leuk Res. 2018;73:58–66.
Ehninger A, Kramer M, Rollig C, Thiede C, Bornhauser M, von Bonin M, et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014;4:e218.
Food and Drug Administration. Highlights of prescribing information: Mylotarg. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761060lbl.pdf. Accessed 27 Nov 2019.
European Medicines Agency. Summary of product characteristics: Mylotarg. https://www.ema.europa.eu/en/documents/product-information/mylotarg-epar-product-information_en.pdf. Accessed 27 Nov 2019.
Castejón N, Cappelleri JC, Cuervo J, Lang K, Mehta P, Mokgokong R, et al. Social preferences for health states associated with acute myeloid leukemia for patients undergoing treatment in the United Kingdom. Health Qual Life Outcomes. 2018;16:66.
National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal. 2013. http://www.nice.org.uk/process/pmg9. Accessed 27 Oct 2017.
Castaigne S, Pautas C, Terre C, Raffoux E, Bordessoule D, Bastie JN, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379:1508–16.
Achcar JA, Coelho-Barros EA, Mazucheli J. Cure fraction models using mixture and non-mixture models. Syst Control Book Ser. 2012;51(1):1–9.
Othus M, Barlogie B, Leblanc ML, Crowley JJ. Cure models as a useful statistical tool for analyzing survival. Clin Cancer Res. 2012;18(14):3731–6.
Jia X, Sima CS, Brennan MS, Panageas KS. Cure models for the analysis of time-to-event data in cancer studies. J Surg Oncol. 2013;108(6):342–7.
Francisci S. Cure models. Presentation made to the European partnership for action against cancer WP9 satellite meeting. January 23, 2014. https://ec.europa.eu/jrc/sites/jrcsh/files/epaac-wp9-session3-francisci.pdf. Accessed 9 Nov 2017.
Peng Y, Dear K, Denham JW. A generalized F mixture model for cure rate estimation. Stat Med. 1998;17:813–30.
Gardin C, Chevret S, Pautas C, Turlure P, Raffoux E, Thomas X, et al. Superior long-term outcome with idarubicin compared with high-dose daunorubicin in patients with acute myeloid leukemia age 50 years and older. J Clin Oncol. 2013;31(3):321–7.
Shah A, Andersson TML, Rachet B, Björkholm M, Lambert PC. Survival and cure of acute myeloid leukaemia in England, 1971–2006: a population-based study. Br J Haematol. 2013;162:509–16.
Muresan B, Mamolo CM, Cappelleri JC, Mokgokong R, Palaka A, Hills RK, et al. Comparison of cure rates between gemtuzumab ozogamicin plus standard of care chemotherapy vs standard of care alone in patients with newly diagnosed acute myeloid leukemia. Blood. 2018;132(Suppl 1):2712.
Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13(5):509–18.
Scottish Medicines Consortium. Defibrotide, 80 mg/mL, concentrate for solution for infusion (Defitelio®). SMC No. (967/14). 2014. https://www.scottishmedicines.org.uk/files/advice/M__Scottish_Medicine_Consortium_Web_Data_Audit_advice_Advice_by_Year_2014_No.6_-_June_2014_defibrotide__Defitelio__FINAL_May_2014_for_website.pdf. Accessed 9 Nov 2017.
National Institute for Health and Care Excellence (NICE). Inotuzumab ozogamicin for treating relapsed or refractory acute lymphoblastic leukaemia [ID893]. Committee Papers. 18 August 2017. https://www.nice.org.uk/guidance/gid-ta10091/documents/committee-papers-2. Accessed 9 Nov 2017.
British National Formulary (BNF) Online. 2017. https://www.medicinescomplete.com/mc/bnf/current/. Accessed 2 Mar 2017.
Department of Health (DoH). Drugs and pharmaceutical electronic market information (eMit). 2017. https://www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit. Accessed 20 July 2017.
Department of Health (DoH). NHS reference costs 2015 to 2016. 2016. https://www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016. Accessed 20 July 2017.
Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB, ISPOR-SMDM Modeling Good Research Practices Task Force. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Value Health. 2012;15(6):843–50.
Latimer N. NICE DSU Technical Support Document No. 14. Survival analysis for economic evaluations alongside clinical trials—extrapolation with patient-level data. Med Decis Mak. 2013;33(6):743–54.
National Institute for Health and Care Excellence. Final appraisal document: gemtuzumab ozogamicin for untreated acute myeloid leukaemia. 2018. https://www.nice.org.uk/guidance/ta545/documents/final-appraisal-determination-document. Accessed 27 Nov 2019.
Office for National Statistics (ONS). National Life Tables, England and Wales. 2016. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesenglandandwalesreferencetables. Accessed 20 July 2017.
British National Formulary (BNF) Online. 2020. https://www.medicinescomplete.com/#/content/bnf/_504336411. Accessed 7 Oct 2020.
National Health Service Blood and Transplant (NHSBT). Price list 2016/2017. http://hospital.blood.co.uk/media/28230/component-price-list-2016-2017.pdf. Accessed 20 July 2017.
National Health Service Blood and Transplant (NHSBT). Unrelated donor stem cell transplantation in the UK. 2014. http://www.nhsbt.nhs.uk/download/unrelated_donor_stem_cell_transplantation_in_the_uk.pdf. Accessed 20 July 2017.
Espérou H, Brunot A, Roudot-Thoraval F, Buzyn A, Dhedin N, Rio B, et al. Predicting the costs of allogeneic sibling stem-cell transplantation: results from a prospective, multicenter, French study. Transplantation. 2004;77(12):1854–8.
Curtis L, Burns A. Personal Social Services Research Unit (PSSRU). Unit costs of health and social care 2016. http://www.pssru.ac.uk/project-pages/unit-costs/2016/. Accessed 20 July 2017.
Addicott R, Dewar S. Improving choice at end of life. King's Fund. 2008. http://www.kingsfund.org.uk/sites/files/kf/improving-choice-end-of-lifedescriptive-analysis-impact-costs-marie-curie-choice-programme-lincolnshirerachael-addicot-steve-dewar-april-2008.pdf. Accessed 23 Aug 2017.
National Institute for Health and Care Excellence. Company submission: Azacitidine for treating acute myeloid leukaemia with more than 30% bone marrow blasts. 2016. https://www.nice.org.uk/guidance/ta399/documents/committee-papers. Accessed 15 June 2020.
Kurosawa S, Yamaguchi H, Yamaguchi T, Fukunaga K, Yui S, Wakita, et al. Decision analysis of postremission therapy in cytogenetically intermediate-risk acute myeloid leukemia: the impact of FLT3 internal tandem duplication, nucleophosmin, and CCAAT/enhancer binding protein alpha. Biol Blood Marrow Transplant. 2016; 6(22):1125–32.
McKenzie L, van der Pol M. Mapping the EORTC QLQ C-30 onto the EQ-5D instrument: the potential to estimate QALYs without generic preference data. Value Health. 2009;12(1):167–71.
National Institute for Health and Care Excellence (NICE). Midostaurin for untreated acute myeloid leukaemia. Technology appraisal guidance 523. 13 June 2018. https://www.nice.org.uk/guidance/ta523. Accessed 10 Mar 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was conducted by RTI Health Solutions (RTI-HS) under the direction of Pfizer Inc and was funded by Pfizer Inc. The authors wish to thank Dr. Hervé Dombret and Dr. Sylvie Castaigne for their expertise on the ALFA-0701 study and AML; Ingress-Health for performing survival analyses of the ALFA-0701 study data; and the clinical, statistical, and health economics experts who provided input into the model assumptions, design, and validation (Professor Nigel Russell, Professor Richard Clark, Dr. Paul Cahalin, Dr. Dominic Culligan, Dr. Sahra Ali, Dr. Priyanka Mehta, Dr. Victoria Potter, Professor Robert Hills, Professor Alan Brennan, Dr Nicholas Latimer, and Dr Praveen Thokala).
Conflict of interest
Alexander Russell-Smith and Carla Mamolo are paid employees of Pfizer Inc, USA. James Brockbank and Christopher Knight are employees of RTI-HS, who were paid consultants to Pfizer in connection with the development of this manuscript.
Availability of data and materials
The authors confirm that the key data supporting the findings of this study are available within the article and its supplementary materials. Additional data are available from the corresponding author, Alexander Russell-Smith, upon reasonable request.
Author contributions
All authors contributed to the study conception and design. Material preparation, non-clinical data collection and analysis were performed by JB and CK. The first draft of the manuscript was written by JB and CK, and all authors commented on previous versions of the manuscript. All authors reviewed and approved the final manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Russell-Smith, T.A., Brockbank, J., Mamolo, C. et al. Cost Effectiveness of Gemtuzumab Ozogamicin in the First-Line Treatment of Acute Myeloid Leukaemia in the UK. PharmacoEconomics Open 5, 677–691 (2021). https://doi.org/10.1007/s41669-021-00278-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-021-00278-3