Abstract
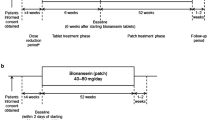
Asenapine, an atypical antipsychotic indicated for the treatment of schizophrenia, was first available as a twice-daily sublingual tablet. However, there are some challenges associated with the use of sublingual asenapine. More recently, a comparable transdermal formulation of asenapine (Secuado®) has been approved in the USA as the first antipsychotic patch for the treatment of schizophrenia in adults. Asenapine transdermal delivery system (TDS) offers a simpler and more convenient dosage regimen and avoids adverse events related to sublingual administration, potentially leading to better adherence to treatment. In a pivotal 6-week phase 3 study in patients with an acute exacerbation of schizophrenia, once-daily application of asenapine TDS 3.8 mg/24 h and 7.6 mg/24 h significantly reduced psychotic symptoms and global disease severity compared with placebo. Asenapine TDS is generally well tolerated, with the systemic safety profile being largely consistent with that established for sublingual asenapine. Although the use of asenapine TDS is associated with application site reactions, these reactions are generally mild to moderate in severity.
Similar content being viewed by others
References
Keepers GA, Fochtmann LJ, Anzia JM, et al. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 2020;177(9):868–72.
Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull. 2015;114(1):169–79.
Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757–77.
Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adher. 2020;14:1541–51.
Suresh A, Narayan R, Nayak UY. Recent advances in the development of asenapine formulations. Expert Opin Drug Deliv. 2020;17(10):1377–93.
Plosker GL, Deeks ED. Asenapine: a review in schizophrenia. CNS Drugs. 2016;30(7):655–66.
Citrome L, Zeni CM, Correll CU. Patches: established and emerging transdermal treatments in psychiatry. J Clin Psychiatry. 2019;80(4):e1–10.
US FDA. Secuado® (asenapine) transdermal system: US prescribing information. 2020. https://www.dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=685eaf44-5944-4f38-afba-0a4fc0b3462b. Accessed 26 Feb 2021.
Chapel S, Hutmacher MM, Haig G, et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol. 2009;49(11):1297–308.
Suzuki K, Castelli M, Komaroff M, et al. Pharmacokinetic profile of the asenapine transdermal system (HP-3070). J Clin Psychopharmacol. 2021. https://doi.org/10.1097/JCP.0000000000001383.
Center for Drug Evaluation and Research. NDA/BLA multi-disciplinary review and evaluation NDA 212268 Secuado (asenapine) transdermal system 2018. https://www.accessdata.fda.gov/. Accessed 26 Feb 2021.
Citrome L, Walling D, Zeni C, et al. Efficacy and safety of HP-3070, an asenapine transdermal system, in patients with schizophrenia: a phase 3, randomized, placebo-controlled study. J Clin Psychiatry. 2020;82(1):20m13602.
Citrome L, Walling D, Zeni C, et al. Efficacy and safety of an asenapine transdermal patch (asenapine transdermal system, HP-3070) in the treatment of adults with schizophrenia: a phase 3, randomized, double-blind, placebo-controlled, 6-week inpatient study [abstract no. T213]. Neuropsychopharmacology. 2018;43(Suppl):S343–4.
Ayyagari R, Thomason D, Mu F, et al. Association of antipsychotic treatment switching in patients with schizophrenia, bipolar, and major depressive disorders. J Med Econ. 2020;23(2):204–12.
Abruzzo A, Cerchiara T, Luppi B, et al. Transdermal delivery of antipsychotics: rationale and current status. CNS Drugs. 2019;33(9):849–65.
Acknowledgements
The manuscript was reviewed by: J.R. Brašić, Division of Nuclear Medicine and Molecular Imaging, The Russell H. Morgan Department of Radiology and Radiological Science/The Johns Hopkins University School of Medicine, Johns Hopkins Outpatient Center, Baltimore, MD, USA; J. M. Davis, Department of Psychiatry, University of Illinois, Chicago, IL/John Hopkins School of Medicine, Baltimore, MD, USA. During the peer review process, Noven Pharmaceuticals, the marketing-authorization holder of asenapine TDS was offered an opportunity to provide a scientific accuracy review of their data. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflicts of interest
Y.-A. Heo is a salaried employee of Adis International Ltd/Springer Nature, is an editor of Drugs & Therapy Perspectives, was not involved in any publishing decision for the manuscript, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Rights and permissions
About this article
Cite this article
Heo, YA. Asenapine transdermal delivery system (Secuado®) in schizophrenia: a profile of its use in the USA. Drugs Ther Perspect 37, 229–235 (2021). https://doi.org/10.1007/s40267-021-00834-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-021-00834-1