Abstract
Background
A family/personal history of breast, ovarian, or pancreatic cancer is a useful predictive marker for response to platinum-based chemotherapy in treating patients with pancreatic cancer. These cancers, and prostate cancer, are known as BRCA-related malignancies. We evaluated the efficacy of gemcitabine plus oxaliplatin (GEMOX) in patients with metastatic pancreatic cancer with a family/personal history of these cancers.
Methods
Chemotherapy-naïve patients with metastatic pancreatic cancer with a family history of pancreatic/breast/ovarian/prostate cancer or a personal history of breast/ovarian/prostate cancer were included. Patients received fixed dose-rate gemcitabine (1000 mg/m2) and oxaliplatin (100 mg/m2) every 2 weeks. The primary endpoint was 1-year survival, and the threshold and expected values were set at 30 and 50%, respectively. The target sample size was determined to be 43, with a one-sided alpha value of 5% and power of 80%. A total of 45 patients were enrolled.
Results
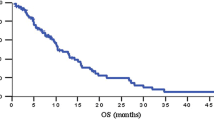
Among the first 43 enrolled patients, the 1-year survival rate was 27.9% [90% confidence interval (CI) 17.0–41.3], which did not meet the primary endpoint. Median overall survival, progression-free survival, and response rates were 7.6 months (95% CI 6.0–10.7), 4.0 months (95% CI 2.0–4.6), and 26.7% (95% CI 14.6–41.9), respectively, in all registered patients. The GEMOX regimen was generally tolerated; the most common grade three or higher adverse events were hematological toxicities.
Conclusion
GEMOX did not show the expected efficacy in patients with metastatic pancreatic cancer with a family or personal history of pancreatic/breast/ovarian/prostate cancer. Selection of GEMOX based on family/personal history is not recommended.
Trial registration number
UMIN000017894.



Similar content being viewed by others
References
Matsubayashi H, Takaori K, Morizane C et al (2017) Familial pancreatic cancer: concept, management and issues. World J Gastroenterol 23:935–948
Klein AP, Brune KA, Petersen GM et al (2004) Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res 64:2634–2638
Pennington KP, Walsh T, Harrell MI et al (2014) Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res 20:764–775
Zhao EY, Shen Y, Pleasance E et al (2017) Homologous recombination deficiency and platinum-based therapy outcomes in advanced breast cancer. Clin Cancer Res 23:7521–7530
Pomerantz MM, Spisák S, Jia L et al (2017) The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer. Cancer 123:3532–3539
Fogelman D, Sugar EA, Oliver G et al (2015) Family history as a marker of platinum sensitivity in pancreatic adenocarcinoma. Cancer Chemother Pharmacol 76:489–498
Golan T, Kanji ZS, Epelbaum R et al (2014) Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 111:1132–1138
Kondo T, Kanai M, Kou T et al (2018) Association between homologous recombination repair gene mutations and response to oxaliplatin in pancreatic cancer. Oncotarget 9:19817–19825
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Okusaka T, Ikeda M, Fukutomi A et al (2014) Phase II study of FOLFIRINOX for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Sci 105:1321–1326
Heinemann V, Quietzsch D, Gieseler F et al (2006) Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 24:3946–3952
Colucci G, Labianca R, Di Costanzo F et al (2010) Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol 28:1645–1651
Poplin E, Feng Y, Berlin J et al (2009) Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the eastern cooperative oncology group. J Clin Oncol 27:3778–3785
Louvet C, Labianca R, Hammel P et al (2005) Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 23:3509–3516
Tempero M, Plunkett W, Ruiz Van Haperen V et al (2003) Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol 21:3402–3408
Ohkawa S, Okusaka T, Isayama H et al (2015) Randomised phase II trial of S-1 plus oxaliplatin vs S-1 in patients with gemcitabine-refractory pancreatic cancer. Br J Cancer 112:1428–1434
Xiong HQ, Varadhachary GR, Blais JC et al (2008) Phase 2 trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer 113:2046–2052
Oettle H, Riess H, Stieler JM et al (2014) (2014) Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 32:2423–2429
Gill S, Ko YJ, Cripps C et al (2016) PANCREOX: a randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol 34:3914–3920
Von Hoff DD, Ervin T, Arena FP et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703
Ueno H, Okusaka T, Funakoshi A et al (2007) A phase II study of weekly irinotecan as first-line therapy for patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 59:447–454
Ueno H, Okusaka T, Ikeda M et al (2007) Phase II study of combination chemotherapy with gemcitabine and cisplatin for patients with metastatic pancreatic cancer. Jpn J Clin Oncol 37:515–520
Okusaka T, Funakoshi A, Furuse J et al (2008) A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol 61:615–621
Ueno H, Okusaka T, Furuse J et al (2011) Multicenter phase II study of gemcitabine and S-1 combination therapy (GS Therapy) in patients with metastatic pancreatic cancer. Jpn J Clin Oncol 41:953–958
NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 2.2014
Takai E, Yachida S, Shimizu K et al (2016) Germline mutations in Japanese familial pancreatic cancer patients. Oncotarget 7:74227–74235
NCCN Clinical Practice Guidelines in Oncology (2020). Pancreatic adenocarcinoma. Version 1. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed 4 Jan 2020
Waddell N, Pajic M, Patch AM et al (2015) Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518:495–501
Sehdev A, Gbolahan O, Hancock BA et al (2018) Germline and somatic DNA damage repair gene mutations and overall survival in metastatic pancreatic adenocarcinoma patients treated with FOLFIRINOX. Clin Cancer Res 24:6204–6211
Golan T, Hammel P, Reni M et al (2019) Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 381:317–327
Holter S, Borgida A, Dodd A et al (2015) Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol 33:3124–3129
Acknowledgements
We wish to thank all the patients and their families for participating in this study. We are grateful to all the investigators in this trial. This research was partially supported by the Practical Research for Innovative Cancer Control from Japan Agency for Medical Research and development, AMED.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Provision of material or patients, data collection and analysis were performed by all authors. The first draft of the manuscript was written by NO, CM, SN, and JF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Morizane C. received research funding from Yakult Honsha, and Eli Lilly Japan. Satake H. received honoraria from Eli Lilly Japan. Mizuno N. received research funding from Dainippon Sumitomo Pharma, MSD, ASLAN Pharmaceuticals, Incyte, and Yakult Honsha. Kanai M received from honoraria from Chugai Pharmaceutical. Shimizu S. received research funding from Yakult Honsha.Ikeda M. received honoraria from Eli Lilly Japan, and received research funding from Yakult Honsha. Okusaka T. received research funding from AstraZeneca, Chugai Pharmaceutical, Eisai, Novartis Pharma, and Bristol-Myers. Furuse J. received honoraria and research funding from Yakult Honsha, and Eli Lilly Japan. The other authors declare that they have no conflict of interest.
Ethical approval
The study was performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its later amendments. The study was approved by the institutional review board of each study site, and all the patients provided informed consent prior to their inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Okano, N., Morizane, C., Nomura, S. et al. Phase II clinical trial of gemcitabine plus oxaliplatin in patients with metastatic pancreatic adenocarcinoma with a family history of pancreatic/breast/ovarian/prostate cancer or personal history of breast/ovarian/prostate cancer (FABRIC study). Int J Clin Oncol 25, 1835–1843 (2020). https://doi.org/10.1007/s10147-020-01721-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01721-x