Abstract
Background:
Hydroxychloroquine (HCQ) maculopathy is irreversible; primary prevention is done by regular monitoring. Guidelines of the Royal College of Ophthalmologists identify definite toxicity as having abnormal results of two screening tests, we present a quantitative method for interpreting these guidelines.
Methods:
We obtained ocular coherence tomography (OCT) scans of 100 patients who have been on HCQ for 5 years or more (patients) and 70 age-matched controls. Both groups had 10’2 visual field (VF) test. We used linear regression to determine the cut-off points for each of the eight Early Treatment of Diabetic Retinopathy Study (ETDRS) macular sectors for the VF and OCT. We calculated the probability of developing maculopathy using logistic regression.
Results:
Mean patient age: 59.9 years, 85% females, no statistically significant age difference between the patients and the control groups. Diagnosis: 64% rheumatoid arthritis, 14% Sjogren’s syndrome, 16% systemic lupus and 6% various other rheumatology conditions. Mean duration of use was 6.3 years. Logistic regression results show strong negative correlation between the outer nuclear layer (ONL) volume and probability of toxicity. Goodness of fit was tested using Hosmer and Lemeshow test that indicates a high significance with a high P-value of 1.
Conclusions:
Combining the ONL volume reduction and VF retinal sensitivity reduction per each of the eight ETDRS macular sectors provides an accurate and objective way of diagnosing HCQ maculopathy, this helps busy eye units establishing an optometrist-led or virtual service because it is independent of the assessor’s level of experience.
Similar content being viewed by others
Introduction
Hydroxychloroquine (HCQ) is widely prescribed by rheumatologists for the treatment of many autoimmune disorders, such as rheumatoid arthritis (RA), cutaneous and systemic lupus erythematosus [1]. Many landmark rheumatological studies have shown that discontinuation of HCQ was associated with threefold increase in the risk of lupus exacerbation, as well as reduction in disease survival rates [2]. In the United Kingdom, ~21,000 patients were started on HCQ between 2007 and 2016, of those, 78% are females and it was reported that 47% were excess-dosed [2].
Understanding the pharmacokinetics of HCQ is essential for correctly diagnosing and predicting the development of maculopathy. The drug is a 4-aminoquinolone that is highly water-soluble, it is absorbed from the gastrointestinal tract and is sequestrated in peripheral tissues with a very high volume of distribution [3].
A recent review of 565 patients taking HCQ by Browning and Chong has concluded that short asthenic women are at higher risk of retinopathy [4]. It has been noticed that the characteristic Bull’s eye maculopathy pattern doesn’t always develop in Asian patients, instead, a pericentral pattern of retinal damage has been noticed [5].
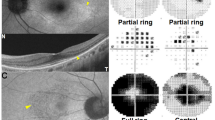
In February 2018, the Royal College of Ophthalmologists published new guidelines for HCQ retinopathy screening, the guidelines recommended the use of visual field (VF) 10’2 test and the spectral domain ocular coherence tomography (SD-OCT) scans of the macula [6]. The reported patterns of VF damage with prolonged HCQ use include: early features, such as reduction of the retinal points sensitivity [7], development of superonasal defects secondary to inferotemporal macular damage [6] and the end-stage of damage that is development of a paracentral ring scotoma [5,6,7].
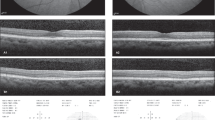
Early signs of maculopathy on SD-OCT scans include the loss of the outer nuclear layer (ONL) that leads to collapse of the perifoveal retina, giving the fovea the characteristic ‘flying saucer’ appearance [8] (Fig. 1), advanced maculopathy has been described as loss of the photoreceptor inner–outer segments and atrophy of the RPE [6, 8]. It was been found that structural changes in the ONL directly correlate to functional changes detectable by multifocal electroretinogram (mfERG) [9]. The 6 × 6 mm macular volume scan that is segmented into retinal layers and overlaid on the three concentric circles of the Early Treatment of Diabetic Retinopathy Study (ETDRS) sectors was found to be accurate in detecting ONL volume loss in patients taking HCQ [10].
En face OCT scans of the macula are colour-coded with the thicker areas being represented by white and red, and the thinner areas are in blue or dark grey colours. a The normal appearance of the ONL on both the en face view, as well as the cross section through the fovea. b Early HCQ toxicity shows ONL thinning in the en face view, but this is not seen in the foveal cross section. c Advanced HCQ toxicity visible in both the en face view, as well as the foveal cross section (flying saucer sign).
Materials and methods
Objectives
-
a.
To establish the graphical distribution of both the ONL volume and the VF-retinal thresholds (RT) per retinal ETDRS sector.
-
b.
To define a cut-off point for toxicity for the both ONL volume and VF-RT per ETDRS sector.
-
c.
To establish the correlation between the ONL volume and VF-RT.
Study design
This retrospective case note review was conducted at the Ophthalmology Department of the Great Western Hospital; a District General hospital located in Swindon town in South West England. A total of 170 individuals have been recruited for the study, 100 of them took HCQ at minimum dose of 400 mg per day for at least 5 years (patients), and the remaining 70 never took the drug (normal subjects) and were specifically recruited for the purpose of the study.
Data collection
As part of the local screening protocol, all study candidates had a macular SD-OCT scan using a Spectralis® OCT scanner (Heidelberg Engineering—Heidelberg, Germany), the scanning protocol used was the 49-line high definition mode that produced a 6 × 6 mm macular cube. We used the Heidelberg Eye Explorer version 2.0 software (HEYEX®) segmentation feature to calculate the ONL volume, the result is displayed as a colour-coded macular map, the thinner areas appear blue or grey, while the thicker areas appear yellow or red, the volume of each of the ETDRS sectors is also displayed in cubic millimetres (mm3). All study candidates also had a central 10’2 VF test using the Humphrey® Field Analyser (Carl Zeiss—Jena, Germany) with a white stimulus; this being the second component of the local screening protocol. Each 1 degree from fixation corresponds to ~288 µm from the foveal centre [11], the 10 degrees on either side of central fixation equal 2.88 mm from the foveal centre; therefore, the whole of the VF plot is represented within the 6 mm ETDRS concentric circles. We overlaid the total deviation values of all 68 retinal points within the 10’2 field onto the concentric ETDRS circles.
Data entry and analysis
We designed a data collection sheet using Microsoft Excel 365 (Microsoft Corporation, Redmond, Washington, USA), the entries include the patient’s anonymous code, age, gender, duration of HCQ use, ONL volume and VF-RT for each of the ETDRS quadrants, i.e., superior, temporal, nasal and inferior (each sector subdivided into para-foveal and peripheral sectors). The data sheet was then programmed to calculate the mean and standard deviation for each of the eight ONL volume sectors and their corresponding VF-RT sectors. The eight sectors are named as follows: superior para-foveal (sector-1), superior peripheral (sector-2), temporal para-foveal (sector-3), temporal peripheral (sector-4), inferior para-foveal (sector-5), inferior peripheral (sector-6), nasal para-foveal (sector-7) and nasal peripheral (sector-8).
Calculation of the cut-off values
We determined the cut-off point for each of the eight retinal sectors (for both ONL volume as well as the corresponding VF-RT value), the cut-off point was determined as the point beyond which only 5% of the normal subjects would test as abnormal, i.e., having a VF-RT or a very thin ONL. Then, we programmed the data sheet to automatically highlight any sectors that are below the calculated cut-off points, a true positive means that the VF-RT is below the cut-off and a test positive means that the ONL volume is below the cut-off. The following values were calculated automatically and in real time: the percentage of eyes below the cut-off point, percentage of agreement between the ONL volume and VF-RT cut-off points, sensitivity and specificity.
Binary logistic regression
The two possible outcomes (toxicity VS no toxicity) were tested using the Statistical Package for Social Sciences version 24 (IBM Corporation, Armonk, New York, USA), with ONL volume and VF-RT as covariates. The following parameters were determined: predictive power of the model, accuracy of prediction, correlation coefficients, probability of toxicity based on ONL volume loss and retinal sectors with the highest predictive value.
Inclusion and exclusion criteria
Patients below the age of 30 and above the 80 years were excluded, all eyes with any macular co-pathology (e.g., dry macular degeneration, macular oedema, diabetic maculopathy, epiretinal membrane, etc.) were excluded. Presence or absence of maculopathy was not among the inclusion criteria. All VFs with false negative error >15%, false positive error >10% or any fixation loss were excluded, and the test was repeated.
Limitations
ONL segmentation was challenging for two high myopic eyes that required manual segmentation. Eyes with other macular pathology, such as cystoid macular oedema were also not suitable for the purpose of this study, we accounted for that by excluding eyes with any other macular pathology.
Results
In both the patients and the normal cohorts, females were a majority (85% and 80%, respectively). Mean age of patients (±1 standard deviation) was 59.9 (±12.6 years), normal cohort 56.3 (±10.5 years). The two groups are normally distributed (D’Agostino–Pearson omnibus test p-value = 0.24), variances of the two groups are homogenous (Levene’s test F = 0.68 and p = 0.05) and there is no statistical difference between the mean ages of the two groups using the independent t-test (p = 0.05), these figures are also shown in Table 1. Mean duration of HCQ use was 6.3 years. Diagnosis: 68% RA, 14% Sjogren’s syndrome, 16% systemic lupus and 2% various other rheumatology conditions.
The mean ONL volume (±1 standard deviation) for each of the macular segments (mm3) in the normal cohort was as follows: sector-1 (0.11 ± 0.01), sector-2 (0.33 ± 0.03), sector-3 (0.12 ± 0.01), sector-4 (0.31 ± 0.03), sector-5 (0.1 ± 0.02), sector-6 (0.28 ± 0.03), sector-7 (0.11 ± 0.02) and sector-8 (0.3 ± 0.03; Fig. 2). The average VF-RT (±1 standard deviation) for each of the macular segments in the normal cohort was as follows (in decibel): sector-1 (33.29 ± 0.33), sector-2 (32.28 ± 0.38), sector-3 (33.40 ± 0.28), sector-4 (32.12 ± 0.46), sector-5 (32.64 ± 0.47), sector-6 (31.34 ± 0.47), sector-7 (33.45 ± 0.31) and sector-8 (32.56 ± 0.41; Fig. 3).
Box plots showing the distribution of the outer nuclear layer volume in normal subjects (green) compared to patients who have been on hydroxychloroquine (red) for 5 years or more. The bars represent 95% limits, and the dots represent data points outside 95% of the data. The cross within the box represent the mean and the horizontal line within the box represent the median.
Box plots showing the distribution of the average retinal sensitivity in normal subjects (green) compared to patients who have been on hydroxychloroquine (red) for 5 years or more, per each of the areas corresponding the ETDRS sectors. The bars represent 95% limits, and the dots represent data points outside 95% of the data. The cross within the box represent the mean and the horizontal line within the box represent the median.
We used these values from the normal cohort to determine the cut-off point for both the ONL and the corresponding VF-RT in each of the eight macular sectors, these cut-off points were calculated using linear regression and are as follows for the ONL volume (mm3): sector-1 (0.1), sector-2 (0.3), sector-3 (0.11), sector-4 (0.29), sector-5 (0.09), sector-6 (0.29), sector-7 (0.09) and sector-8 (0.27). And for the VF-RT were as follows (in decibel): sector-1 (33.25), sector-2 (32.29), sector-3 (33.41), sector-4 (32.12), sector-5 (32.26), sector-6 (31.34), sector-7 (33.34) and sector-8 (32.56; Fig. 4).
Ninety one percent of the ONL volume, and 86% of the VF-RT of the normal cohort are above the determined cut-off point. The OCT and VF characteristics of the eyes that were identified by our algorithm as positive are shown in Table 2, this is together with a comparison to the criteria of the Royal College of Ophthalmologists.
Most tests of goodness of fit give a large p-value if the fit was good [12]. We tested the goodness of fit of the logistic regression model using the Hosmer and Lemeshow test, the p-value of the test was 1.000 that indicates a good fit [12].
Classification of results in the binary logistic regression model shows 97.7% correct classification rate, which means that ONL volume is a strong predictor of VF-RT loss.
Retinal sectors that have the highest predictive power were found to be: sector-1 (p = 0.04), sector-2 (p = 0.02), sector-6 (p = 0.02) and sector-7 (p = 0.05).
Discussion
Interpretation of VF and SD-OCT results is a subjective process, and can result in inter-observer variation in diagnosing, as well as monitoring HCQ maculopathy [9]. Looking at a single OCT section through the fovea can also result in missing of OCT changes in the adjacent sections. We have designed this study to tackle the subjectivity issue and to provide—for the first time—objective cut-off points that can be used by ophthalmologists or allied health professionals, who will be screening patients for HCQ maculopathy.
Our cut-off points are for diagnosing ‘definite HCQ maculopathy’, this is in-line with the current screening guidelines that define definite HCQ maculopathy, as having abnormal results of two of the screening tests. Our method is easy to implement as most ophthalmology departments already use SD-OCT and 10’2 VFs as screening tools and clinicians are familiar with these test modalities.
HCQ maculopathy is known to be most prevalent in patients who have been on the drug for 5 years or more [6, 8,9,10], we therefore selected a group of patients who have been on HCQ for a minimum of 5 years, and we set our cut-off point to be diagnostic of definite toxicity, both of the tests we used as covariates (SD-OCT and VF-RT) are known to correlate well with toxicity [9]. This will also allow us to have a reference point when we look at test results from any patient, who has taken the drug for a longer or shorter period, and to compare those results to our current data easily.
Once VF defects develop they are known to be irreversible [4, 7, 13], this observation has created a considerable amount of concern across specialties, with a few cases ended up being taken to court [6, 14]. Using logistic regression method enables clinicians to diagnose subclinical VF defects and also to predict the probability of that by giving an odds p-value of toxicity based on inputs from VF-RT and ONL volume. Our model calculates a VF-RT for each of the macular sectors separately, as previous studies have shown that the global VF-RT can miss focal defects [13, 15, 16]. The logistic regression formula produces coefficients that are used in the prediction, and with more data being entered, the relationship between the input and the output data is going to change, changing with it the prediction accuracy. The logistic regression formula automatically updates the coefficients to account for the change in input, making the output prediction constantly accurate [17].
We found certain areas of retinal damage to be most indicative of toxicity, these are sectors 1 (superior para-foveal), 2 (superior peripheral), 6 (inferior para-foveal) and 7 (nasal para-foveal). The association between the superior macular quadrant damage and development of toxicity has been documented in the literature before [18].
Once the RPE is disrupted, retinal and VF changes may continue to progress even after the cessation of HCQ therapy [19], this makes it important to detect retinal toxicity as early as possible so that HCQ can be stopped. The Royal College of Ophthalmologists recommends contacting the rheumatologists with such recommendation as soon as possible [6, 20]. Our aim is also to detect maculopathy well in advance of visible RPE changes, this is achieved by correlating retinal structural changes (seen on OCT) to corresponding areas of reduced retinal sensitivity on VF, which allows for early detection of maculopathy, this has also been pointed out in the guidelines of the American Academy of Ophthalmology (AAO) screening guidelines [21]. Our interpretation of the results of the screening tests is in-line with both guidelines of the AAO and the Royal College of Ophthalmologists.
Sensitivity and specificity of our model (94.4 and 98.9, respectively) exceed those of previously proposed screening tests like microperimetry [13], mfERG [22], high-speed ultra-high-resolution OCT [23] and fundus autofluorescence [24].
Many ocular and extraocular factors are known to potentiate the action of HCQ leading to accelerated toxicity, these include high dose (>5mg/kg/day), dry age-related macular degeneration, concurrent use of tamoxifen and chronic kidney disease [4, 25, 26], it is thus of utmost importance to recognise those cases early and to fast-track such referrals and to monitor these particular cases more closely [27], our model also accounts for this by enabling hospitals to set up a virtual screening service that would be quickly and easily accessible.
This study has proposed an objective and reliable screening method that could revolutionise the current practice, our model incorporates machine learning capabilities and uses artificial intelligence to autocorrect and auto-recalculate the cut-off points to achieve the highest sensitivity and specificity possible. We provide an easy method of calculation that uses the available screening tests and makes the interpretation of the results highly objective, this means that screening is no longer required to be done by doctors and can be run on virtual basis, which will have a great impact on improving efficiency. We are writing a computer software programme that will enable ophthalmologists to use our data in their clinical practice. The software utilises optical character recognition to scan and upload retinal sensitivity threshold points from the VF test report, and the ONL volume from the OCT report. These data are then analysed with reference to the predefined cut-off points, and a result is made available instantly.
Summary table
What was known before
-
Prevalence of HCQ maculopathy was reported to be 7.5% in patients who take HCQ for >5 years.
What this study adds
-
Prevalence reported by this is study is approaching 16%, this is due to the ability to diagnose maculopathy at an earlier stage.
References
Yusuf IH, Sharma S, Luqmani R, Downes SM. Hydroxychloroquine retinopathy. Eye. 2017;4:828–45.
Jorge AM, Melles RB, Zhang Y, Lu N, Rai SK, Young LH, et al. Hydroxychloroquine prescription trends and predictors for excess dosing per recent ophthalmology guidelines. Arthritis Res Ther. 2018;20:133–40.
Browning DJ. Hydroxychloroquine and chloroquine retinopathy. 1st ed. York, UK: Springer; 2014.
Browning DJ, Lee C. Somatotype, the risk of hydroxychloroquine retinopathy, and safe daily dosing guidelines. Clin Ophthalmol. 2018;12:811–8.
Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology 2015;122:110–6.
Lotery A, Yusuf I, Foot B, Bishop P, Burdon M, Watson S, et al. Hydroxychloroquine and chloroquine retinopathy: recommendations on screening. London: The Royal College of Ophthalmologists; 2018.
Anderson C, Blaha GR, Marx JL. Humphrey visual field findings in hydroxychloroquine toxicity. Eye. 2011;25:1535–45.
Chen E, Brown DM, Benz MS, Fish RH, Wong TP, Kim RY, et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the “flying saucer” sign). Clin Ophthalmol. 2010;4:1151–8.
Allahdina AM, Stetson PF, Vitale S, Wong WT, Chew EY, Ferris FL, et al. Optical coherence tomography minimum intensity as an objective measure for the detection of hydroxychloroquine toxicity. Invest Ophthalmol Vis Sci. 2018;59:1953–63.
Ahn SJ, Joung J, Lim HW, Lee BR. Optical coherence tomography protocols for screening of hydroxychloroquine retinopathy in asian patients. Am J Ophthalmol. 2017;9:11–18.
Kolb H, Fernandez E, Nelson R. Webvision: the organization of the retina and visual system [Internet]. Salt Lake City, UT: University of Utah Health Sciences Center; 1995.
Bewick V, Cheek L, Ball J. Statistics review 14: logistic regression. Crit Care. 2005;9:112–8.
Iftikhar M, Kaur R, Nefalar A, Usmani B, Kherani S, Rashid I, et al. Microperimetry as a screening test for hydroxychloroquine retinopathy The Hard-Risk-1 Study. J Retinal Vitreous Dis. 2018;0:1–7.
Tucker WR, Galloway J, Walsh S. The gathering storm: hydroxychloroquine retinopathy screening in the U.K. Br J Dermatol. 2017;176:1420–1.
Espandar G, Moghimi J, Ghorbani R, Pourazizi M, Seiri MA, et al. Retinal toxicity in patients treated with hydroxychloroquine: a cross-sectional study. Med Hypothesis Disco Innov Ophthalmol. 2016;5:41–46.
Iselin KC, Marti P, Pless M. Hydroxychloroquine-induced retinal toxicity. Klin Monatsbl Augenheilkd. 2016;233:514–6.
Brownlee J. Logistic regression for machine learning. Machine Learning Mastery Pty. Ltd., Australia. 2016. https://machinelearningmastery.com/logistic-regression-for-machine-learning/. Accessed 01 April 2016.
Babeau F, Busetto T, Hamel C, Villain M, Daien V. Adaptive optics: a tool for screening hydroxychloroquine-induced maculopathy? Acta Ophthalmol. 2017;14:424–5.
Lally DR, Heier JS, Baumal C, Witkin AJ, Maler S, Shah CP, et al. Expanded spectral domain-OCT findings in the early detection of hydroxychloroquine retinopathy and changes following drug cessation. Int J Retin Vitr. 2016;2:1–11.
Khaleel A. Risk of ophthalmologic complications as a result of hydroxychloroquine therapy. Ann Rheum Dis. 2018;6:693–4.
Marmor M, Kellner U, Lai T, Melles R, Mieler W. Recommendations on screenig for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386–94.
Ruberto G, Bruttini C, Tinelli C, Cavagna L, Bianchi A, Milano G. Early morpho-functional changes in patients treated with hydroxychloroquine: a prospective cohort study. Graefes Arch Clin Exp Ophthalmol. 2018;256:2201–10.
Rodriguez-Padilla JA, Hedges TR, Monson B, Srinivasan V, Wojtkowski M, Reichel E, et al. High-speed ultra-high-resolution optical coherence tomography findings in hydroxychloroquine retinopathy. Arch Ophthalmol. 2007;125:775–80.
Hansen M, Schuman S. Hydroxychloroquine-induced retinal toxicity. EyeNet Mag. 2011;76:33–35.
Smith GP. High-risk category for early annual ophthalmology screening of patients receiving hydroxychloroquine. J Am Acad Dermatol. 2017;7:171.
Stern EM, Johnson JS, Mazzulla DA. Highly accelerated onset of hydroxychloroquine macular retinopathy. Ochsner J. 2017;17:280–3.
Zaidi FH, Rennie CA, Drinkwater AK, Sahu D, Akyol E, Lotery AJ. How to set up a hydroxychloroquine retinopathy screening service. Eye. 2019;33:1679–82.
Acknowledgements
The authors would like to extend their thanks to the staff of the Eye Clinic at the Great Western Hospital in Swindon for their support of this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hasan, H., Lotery, A., Price, E.J. et al. An objective method of diagnosing hydroxychloroquine maculopathy. Eye 35, 1922–1929 (2021). https://doi.org/10.1038/s41433-020-01174-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01174-6
This article is cited by
-
Hydroxychloroquine
Reactions Weekly (2021)