Abstract
Purpose of Review
Despite broad uptake of antiretroviral therapy (ART), tuberculosis (TB) incidence and mortality among people with HIV remain unacceptably high. Short-course regimens for TB, incorporating both novel and established drugs, offer the potential to enhance adherence and completion rates, thereby reducing the global TB burden. This review will outline short-course regimens for TB among patients with HIV.
Recent Findings
After many years without new agents, there is now active testing of many novel drugs to treat TB, both for latent infection and active disease. Though not all studies have included patients with HIV, many have, and there are ongoing trials to address key implementation challenges such as potent drug-drug interactions with ART.
Summary
The goal of short-course regimens for TB is to enhance treatment completion without compromising efficacy. Particularly among patients with HIV, studying these shortened regimens and integrating them into clinical care are of urgent importance. There are now multiple short-course regimens for latent infection and active disease that are safe and effective among patients with HIV.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• World Health Organization (WHO). Global Tuberculosis Report 2019. World Health Organization; 2019. Current data on the state of the global TB epidemic.
Séraphin MN, Hsu H, Chapman HJ, de Andrade Bezerra JL, Johnston L, Yang Y, et al. Timing of treatment interruption among latently infected tuberculosis cases treated with a nine-month course of daily isoniazid: findings from a time to event analysis. BMC Public Health. 2019;19(1):1214. https://doi.org/10.1186/s12889-019-7524-4.
Clinical Pipeline. Working Group on New TB Drugs. https://www.newtbdrugs.org/pipeline/clinical. .
Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. https://doi.org/10.1371/journal.pmed.1002152.
Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1999;340(5):367–73. https://doi.org/10.1056/NEJM199902043400507.
•• González Fernández L, Casas EC, Singh S, et al. New opportunities in tuberculosis prevention: implications for people living with HIV. J Int AIDS Soc. 2020;23(1):e25438. https://doi.org/10.1002/jia2.25438. This paper offers a comprehnsive review of TB prevention in HIV.
Comstock, Ferebee GW, Shirley H, Hammes LM. A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis. 1967;95(6):935–43. https://doi.org/10.1164/arrd.1967.95.6.935.
Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;9(1):CD000171. https://doi.org/10.1002/14651858.CD000171.pub3.
Danel C, Moh R, Gabillard D, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–22. https://doi.org/10.1056/NEJMoa1507198.
• Badje A, Moh R, Gabillard D, et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health. 2017;5(11):e1080–9. https://doi.org/10.1016/S2214-109X(17)30372-8. A report of the first clinical trial showing a mortality benfit for people with HIV from TB preventive therapy.
World Health Organization (WHO). Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva; 2018.
Centers for Disease Control and Prevention (CDC). Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. MMWR Recomm Rep. 2019:424. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
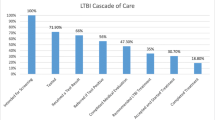
Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(11):1269–78. https://doi.org/10.1016/S1473-3099(16)30216-X.
Stockbridge EL, Miller TL, Carlson EK, Ho C. Predictors of latent tuberculosis infection treatment completion in the US private sector: an analysis of administrative claims data. BMC Public Health. 2018;18(1):662. https://doi.org/10.1186/s12889-018-5578-3.
Horsburgh CR, Goldberg S, Bethel J, et al. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137(2):401–9. https://doi.org/10.1378/chest.09-0394.
LoBue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med. 2003;168(4):443–7. https://doi.org/10.1164/rccm.200303-390OC.
Robert M, Todd J, Ngowi BJ, et al. Determinants of isoniazid preventive therapy completion among people living with HIV attending care and treatment clinics from 2013 to 2017 in Dar es Salaam Region, Tanzania. A cross-sectional analytical study. BMC Infect Dis. 2020;20(1):276. https://doi.org/10.1186/s12879-020-04997-6.
Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161(4 Pt 2):S221–47. https://doi.org/10.1164/ajrccm.161.supplement_3.ats600.
Gordin F, Chaisson RE, Matts JP, Miller C, de Lourdes Garcia M, Hafner R, et al. Rifampin and pyrazinamide vs isoniazid for prevention of tuberculosis in HIV-infected persons: an international randomized trial. J Am Med Assoc. 2000;283(11):1445–50. https://doi.org/10.1001/jama.283.11.1445.
Halsey NA, Coberly JS, Desormeaux J, Losikoff P, Atkinson J, Moulton LH, et al. Randomised trial of isoniazid versus rifampicin and pyrazinamide for prevention of tuberculosis in HIV-1 infection. Lancet. 1998;351(9105):786–92. https://doi.org/10.1016/S0140-6736(97)06532-X.
Martínez Alfaro E, Cuadra F, Solera J, Ángel Maciá M, Geijo P, Antonio Sánchez Martínez P, et al. Evaluación de dos pautas de quimioprofilaxis tuberculosa en pacientes infectados por el virus de la inmunodeficiencia humana. Med Clin (Barc). 2000;115(5):161–5. https://doi.org/10.1016/S0025-7753(00)71496-5.
Rivero A, López-Cortés L, Castillo R, Lozano F, Ángel García M, Díez F, et al. Ensayo clínico aleatorizado de tres pautas de quimioprofilaxis para prevenir la tuberculosis en pacientes infectados por el VIH con anergia cutánea. Enferm Infecc Microbiol Clin. 2003;21(6):287–92. https://doi.org/10.1016/S0213-005X(03)72942-5.
Rivero A, López-Cortés L, Castillo R, Verdejo J, Ángel García M, Javier Martínez-Marcos F, et al. Ensayo clínico aleatorizado para evaluar tres pautas cortas de tratamiento de la infección latente tuberculosa en pacientes infectados por el VIH. Enferm Infecc Microbiol Clin. 2007;25(5):305–10. https://doi.org/10.1157/13102265.
Lim HJ, Okwera A, Mayanja-Kizza H, Ellner JJ, Mugerwa RD, Whalen CC. Effect of tuberculosis preventive therapy on HIV disease progression and survival in HIV-infected adults. HIV Clin Trials. 2006;7(4):172–83. https://doi.org/10.1310/hct0704-172.
Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155–66. https://doi.org/10.1056/NEJMoa1104875.
• Sterling TR, Scott NA, Miro JM, et al. Three months of weekly rifapentine and isoniazid for treatment of Mycobacterium tuberculosis infection in HIV-coinfected persons. AIDS. 2016;30(10):1607–15. https://doi.org/10.1097/QAD.0000000000001098. This study documented efficacy of 3HP regimen in patients with HIV.
• Swindells S, Ramchandani R, Gupta A, et al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med. 2019;380(11):1001–11. https://doi.org/10.1056/NEJMoa1806808. Report of a large, phase 3 trial in patients with HIV showing effiacy of 1HP.
Menzies D, Adjobimey M, Ruslami R, Trajman A, Sow O, Kim H, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018;379(5):440–53. https://doi.org/10.1056/NEJMoa1714283.
Churchyard G, Cardenas V, Chihota V, et al. Effectiveness of 3HP annually vs once for HIV-positive people: the WHIP3TB trial. In: Conference on Retrovirus and Opportunistic Infections (CROI). ; 2020.
Dorman SE, Johnson JL, Goldberg S, Muzanye G, Padayatchi N, Bozeman L, et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med. 2009;180(3):273–80. https://doi.org/10.1164/rccm.200901-0078OC.
Jindani A, Harrison TS, Nunn AJ, Phillips PPJ, Churchyard GJ, Charalambous S, et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med. 2014;371(17):1599–608. https://doi.org/10.1056/NEJMoa1314210.
Merle CS, Fielding K, Sow OB, Gninafon M, Lo MB, Mthiyane T, et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med. 2014;371(17):1588–98. https://doi.org/10.1056/NEJMoa1315817.
Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR, et al. Four-month Moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med. 2014;371(17):1577–87. https://doi.org/10.1056/NEJMoa1407426.
Tweed CD, Dawson R, Burger DA, Conradie A, Crook AM, Mendel CM, et al. Bedaquiline, moxifloxacin, pretomanid, and pyrazinamide during the first 8 weeks of treatment of patients with drug-susceptible or drug-resistant pulmonary tuberculosis: a multicentre, open-label, partially randomised, phase 2b trial. Lancet Respir Med. 2019;7:1048–58. https://doi.org/10.1016/S2213-2600(19)30366-2.
Nunn AJ, Phillips PPJ, Meredith SK, Chiang CY, Conradie F, Dalai D, et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med. 2019;380(13):1201–13. https://doi.org/10.1056/NEJMoa1811867.
• Conradie F, Diacon AH, Ngubane N, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382(10):893–902. https://doi.org/10.1056/NEJMoa1901814. Similar report of successful shoter course therapy for XDR TB.
IUAT International Union Against Tb. Efficacy of various durations of isoniazid preventive therapy for tuberculosis : five years of follow-up in the IUAT trial. Bull WHO. 1982;60(4):555–64.
Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm reports Morb Mortal Wkly report Recomm reports. 2020;69(1):1–11. https://doi.org/10.15585/mmwr.rr6901a1
Stagg HR, Zenner D, Harris RJ, Muñoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med. 2014;161(6):419–28. https://doi.org/10.7326/M14-1019.
Whalen CC, Johnson JL, Okwera A, Hom DL, Huebner R, Mugyenyi P, et al. A trial of three regimens to prevent tuberculosis in Ugandan adults infected with the human immunodeficiency virus. N Engl J Med. 1997;337(12):801–8. https://doi.org/10.1056/NEJM199709183371201.
Durovni B, Saraceni V, Moulton LH, Pacheco AG, Cavalcante SC, King BS, et al. Effect of improved tuberculosis screening and isoniazid preventive therapy on incidence of tuberculosis and death in patients with HIV in clinics in Rio de Janeiro, Brazil: a stepped wedge, cluster-randomised trial. Lancet Infect Dis. 2013;13(10):852–8. https://doi.org/10.1016/S1473-3099(13)70187-7.
Chaisson RE, Golub JE. Preventing tuberculosis in people with HIV-no more excuses. Lancet Glob Health. 2017;5(11):e1048–9. https://doi.org/10.1016/S2214-109X(17)30390-X.
Gupta A, Montepiedra G, Aaron L, Theron G, McCarthy K, Bradford S, et al. Isoniazid preventive therapy in HIV-infected pregnant and postpartum women. N Engl J Med. 2019;381(14):1333–46. https://doi.org/10.1056/NEJMoa1813060.
Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the National Tuberculosis Controllers Association and CDC, 2020. Am J Transplant. 2020;20(4):1196–206. https://doi.org/10.1111/ajt.15841.
Maartens G, Boffito M, Flexner CW. Compatibility of next-generation first-line antiretrovirals with rifampicin-based antituberculosis therapy in resource limited settings. Curr Opin HIV AIDS. 2017;12(4):355–8. https://doi.org/10.1097/COH.0000000000000376.
Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed May 5, 2020.
Dooley KE. Short-course rifamycin-based regimens for TB infection (LTBI): why countries should scale up this silver bullet for TB prevention among PLHIV. Boston: TB/HIV Research Meeting Organized by WHO; 2018.
Cerrone M, Alfarisi O, Neary M, Marzinke MA, Parsons TL, Owen A, et al. Rifampicin effect on intracellular and plasma pharmacokinetics of tenofovir alafenamide. J Antimicrob Chemother. 2019;74(6):1670–8. https://doi.org/10.1093/jac/dkz068.
Luetkemeyer AF, Rosenkranz SL, Lu D, Marzan F, Ive P, Hogg E, et al. Relationship between weight, efavirenz exposure, and virologic suppression in HIV-infected patients on rifampin-based tuberculosis treatment in the AIDS clinical trials group A5221 STRIDE study. Clin Infect Dis. 2013;57(4):586–93. https://doi.org/10.1093/cid/cit246.
Cerrone M, Wang X, Neary M, Weaver C, Fedele S, Day-Weber I, et al. Pharmacokinetics of efavirenz 400 mg once daily coadministered with isoniazid and rifampicin in human immunodeficiency virus–infected individuals. Clin Infect Dis. 2019;68(3):446–52. https://doi.org/10.1093/cid/ciy491.
Dooley KE, Kaplan R, Mwelase N, Grinsztejn B, Ticona E, Lacerda M, et al. Dolutegravir-based antiretroviral therapy for patients coinfected with tuberculosis and human immunodeficiency virus: a multicenter, noncomparative, open-label. Randomized Trial Clin Infect Dis. 2019;70(4):549–56. https://doi.org/10.1093/cid/ciz256.
Taburet A-M, Sauvageon H, Grinsztejn B, Assuied A, Veloso V, Pilotto JH, et al. Pharmacokinetics of raltegravir in HIV-infected patients on rifampicin-based antitubercular therapy. Clin Infect Dis. 2015;61(8):1328–35. https://doi.org/10.1093/cid/civ477.
Farenc C, Doroumian S, Cantalloube C, et al. Rifapentine once-weekly dosing effect on efavirenz emtricitabine and tenofovir PKs. In: Conference on Retrovirus and Opportunistic Infections (CROI). Boston; 2014. https://2jg4quetidw2blbbq2ixwziw-wpengine.netdna-ssl.com/wp-content/uploads/sites/2/posters/2014/493.pdf.
Brooks KM, George JM, Pau AK, Rupert A, Mehaffy C, de P, et al. Cytokine-mediated systemic adverse drug reactions in a drug–drug interaction study of dolutegravir with once-weekly isoniazid and rifapentine. Clin Infect Dis. 2018;67(2):193–201. https://doi.org/10.1093/cid/ciy082.
Dooley KE, Savic R, Gupte A, et al. Once-weekly rifapentine and isoniazid for tuberculosis prevention in patients with HIV taking dolutegravir-based antiretroviral therapy: a phase 1/2 trial. Lancet HIV. 2020;3018(20). https://doi.org/10.1016/S2352-3018(20)30032-1
Weiner M, Egelund EF, Engle M, Kiser M, Prihoda TJ, Gelfond JAL, et al. Pharmacokinetic interaction of rifapentine and raltegravir in healthy volunteers. J Antimicrob Chemother. 2014;69(4):1079–85. https://doi.org/10.1093/jac/dkt483.
Podany AT, Bao Y, Swindells S, Chaisson RE, Andersen JW, Mwelase T, et al. Efavirenz pharmacokinetics and pharmacodynamics in HIV-infected persons receiving Rifapentine and isoniazid for tuberculosis prevention. Clin Infect Dis. 2015;61(8):1322–7. https://doi.org/10.1093/cid/civ464.
Podany A. Efavirenz pharmacokinetics in HIV/TB coinfected persons initiating ART while receiving high dose rifapentine. In. 2019; http://regist2.virology-education.com/presentations/2019/20AntiviralPK/07_Podany.pdf.
Svensson EM, Dooley KE, Karlsson MO. Impact of lopinavir-ritonavir or nevirapine on bedaquiline exposures and potential implications for patients with tuberculosis-HIV coinfection. Antimicrob Agents Chemother. 2014;58(11):6406–12. https://doi.org/10.1128/AAC.03246-14.
Brill MJE, Svensson EM, Pandie M, Maartens G, Karlsson MO. Confirming model-predicted pharmacokinetic interactions between bedaquiline and lopinavir/ritonavir or nevirapine in patients with HIV and drug-resistant tuberculosis. Int J Antimicrob Agents. 2017;49(2):212–7. https://doi.org/10.1016/j.ijantimicag.2016.10.020.
Dooley KE, Park J, Swindells S, Allen R, Haas DW, Cramer Y, et al. Safety, tolerability, and pharmacokinetic interactions of the antituberculous agent TMC207 (bedaquiline) with efavirenz in healthy volunteers: AIDS Clinical Trials Group study A5267. J Acquir Immune Defic Syndr. 2012;59(5):455–62. https://doi.org/10.1097/QAI.0b013e3182410503.
Svensson EM, Aweeka F, Park JG, Marzan F, Dooley KE, Karlsson MO. Model-based estimates of the effects of efavirenz on bedaquiline pharmacokinetics and suggested dose adjustments for patients coinfected with HIV and tuberculosis. Antimicrob Agents Chemother. 2013;57(6):2780–7. https://doi.org/10.1128/AAC.00191-13.
Dooley KE, Luetkemeyer AF, Park JG, Allen R, Cramer Y, Murray S, et al. Phase I safety, pharmacokinetics, and pharmacogenetics study of the antituberculosis drug PA-824 with concomitant lopinavir-ritonavir, efavirenz, or rifampin. Antimicrob Agents Chemother. 2014;58(9):5245–52. https://doi.org/10.1128/AAC.03332-14.
Mwinga A, Hosp M, Godfrey-Faussett P, Quigley M, Mwaba P, Mugala BN, et al. Twice weekly tuberculosis preventive therapy in HIV infection in Zambia. Aids. 1998;12(18):2447–57. https://doi.org/10.1097/00002030-199818000-00014.
Jasmer RM, Saukkonen JJ, Blumberg HM, Daley CL, Bernardo J, Vittinghoff E, et al. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann Intern Med. 2002;137(8):640–7. https://doi.org/10.7326/0003-4819-137-8-200210150-00007.
Tortajada C, Martínez-Lacasa J, Sánchez F, et al. Is the combination of pyrazinamide plus rifampicin safe for treating latent tuberculosis infection in persons not infected by the human immunodeficiency virus? Int J Tuberc Lung Dis. 2005;9(3):276–81.
Martinson NA, Barnes GL, Moulton LH, Msandiwa R, Hausler H, Ram M, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365(1):11–20. https://doi.org/10.1056/NEJMoa1005136.
Mathad JS. Rifapentine pharmacokinetics and safety in pregnant women with and without HIV on 3HP. In: Conference on Retrovirus and Opportunistic Infections (CROI). ; 2020. https://impaactnetwork.org/DocFiles/CROI2020/Mathad_IMPAACT2001.presentation_CROI2020_final.pdf.
Johnson KT, Churchyard GJ, Sohn H, Dowdy DW. Cost-effectiveness of preventive therapy for tuberculosis with isoniazid and rifapentine versus isoniazid alone in high-burden settings. Clin Infect Dis. 2018;67(7):1072–8. https://doi.org/10.1093/cid/ciy230.
Zhang T, Zhang M, Rosenthal IM, Grosset JH, Nuermberger EL. Short-course therapy with daily rifapentine in a murine model of latent tuberculosis infection. Am J Respir Crit Care Med. 2009;180(11):1151–7. https://doi.org/10.1164/rccm.200905-0795OC.
Zhang T, Li SY, Williams KN, Andries K, Nuermberger EL. Short-course chemotherapy with TMC207 and rifapentine in a murine model of latent tuberculosis infection. Am J Respir Crit Care Med. 2011;184(6):732–7. https://doi.org/10.1164/rccm.201103-0397OC.
Knight GM, McQuaid CF, Dodd PJ, Houben RMGJ. Global burden of latent multidrug-resistant tuberculosis: trends and estimates based on mathematical modelling. Lancet Infect Dis. 2019;19(8):903–12. https://doi.org/10.1016/S1473-3099(19)30307-X.
Nahid P, Mase SR, Migliori GB, Sotgiu G, Bothamley GH, Brozek JL, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019;200(10):e93–e142. https://doi.org/10.1164/rccm.201909-1874ST.
Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121(6):939–49. https://doi.org/10.1164/arrd.1980.121.6.939.
Fox W, Mitchison DA. Short course chemotherapy for pulmonary tuberculosis. AMERREVRESPDIS. 1975;111(6):845–8. https://doi.org/10.1164/arrd.1975.111.3.325.
World Health Organization (WHO). Guidelines for treatment of drug-susceptible tuberculosis and patient care. Geneva; 2017. https://apps.who.int/iris/bitstream/handle/10665/255052/9789241550000-eng.pdf.
Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147–95. https://doi.org/10.1093/cid/ciw376.
Jawahar MS, Banurekha VV, Paramasivan CN, et al. Randomized clinical trial of thrice-weekly 4-month moxifloxacin or gatifloxacin containing regimens in the treatment of new sputum positive pulmonary tuberculosis patients. Doherty TM, ed. PLoS One. 2013;8(7):e67030. https://doi.org/10.1371/journal.pone.0067030.
Velayutham B, Jawahar MS, Nair D, Navaneethapandian P, Ponnuraja C, Chandrasekaran K, et al. 4-month moxifloxacin containing regimens in the treatment of patients with sputum-positive pulmonary tuberculosis in South India – a randomised clinical trial. Tropical Med Int Health. 2020;25(4):483–95. https://doi.org/10.1111/tmi.13371.
Dorman SE, Nahid P, Kurbatova EV, Goldberg SV, Bozeman L, Burman WJ, et al. High-dose rifapentine with or without moxifloxacin for shortening treatment of pulmonary tuberculosis: study protocol for TBTC study 31/ACTG A5349 phase 3 clinical trial. Contemp Clin Trials. 2020;90(January):105938. https://doi.org/10.1016/j.cct.2020.105938.
Chabala C, Turkova A, Thomason MJ, et al. Shorter treatment for minimal tuberculosis (TB) in children (SHINE): a study protocol for a randomised controlled trial. Trials. 2018;19(1):237. https://doi.org/10.1186/s13063-018-2608-5.
Pietersen E, Ignatius E, Streicher EM, Mastrapa B, Padanilam X, Pooran A, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet. 2014;383(9924):1230–9. https://doi.org/10.1016/S0140-6736(13)62675-6.
Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368(9547):1575–80. https://doi.org/10.1016/S0140-6736(06)69573-1.
O’Donnell MR, Padayatchi N, Kvasnovsky C, Werner L, Master I, Horsburgh CR. Treatment outcomes for extensively drug-resistant tuberculosis and HIV co-infection. Emerg Infect Dis. 2013;19(3):416–24. https://doi.org/10.3201/eid1903.120998.
• Van Deun A, Maug AKJ, Salim MAH, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182(5):684–92. https://doi.org/10.1164/rccm.201001-0077OC. The first report of successful treatment shorteing for MDR TB.
World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. In: WHO Consol Guidel drug-resistant Tuberc Treat; 2019. p. 99. http://apps.who.int/bookorders.
Ndjeka N, Conradie F, Schnippel K, Hughes J, Bantubani N, Ferreira H, et al. Treatment of drug-resistant tuberculosis with bedaquiline in a high HIV prevalence setting: an interim cohort analysis. Int J Tuberc Lung Dis. 2015;19(8):979–85. https://doi.org/10.5588/ijtld.14.0944.
Borisov SE, Dheda K, Enwerem M, et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J. 2017;49(5). https://doi.org/10.1183/13993003.00387-2017.
von Groote-Bidlingmaier F, Patientia R, Sanchez E, Balanag V Jr, Ticona E, Segura P, et al. Efficacy and safety of delamanid in combination with an optimised background regimen for treatment of multidrug-resistant tuberculosis: a multicentre, randomised, double-blind, placebo-controlled, parallel group phase 3 trial. Lancet Respir Med. 2019;2600(18):1–12. https://doi.org/10.1016/S2213-2600(18)30426-0.
Esmail A, Sabur NF, Okpechi I, Dheda K. Management of drug-resistant tuberculosis in special subpopulations including those with HIV co-infection, pregnancy, diabetes, organ-specific dysfunction, and in the critically ill. J Thorac Dis. 2018;10(5):3102–18. https://doi.org/10.21037/jtd.2018.05.11.
WHO operational handbook on tuberculosis (module 1 - preventino): tuberculosis preventive treatment. Geneva; 2020. https://apps.who.int/iris/bitstream/handle/10665/331525/9789240002906-eng.pdf.
Cellamare M, Ventz S, Baudin E, Mitnick CD, Trippa L. A Bayesian response-adaptive trial in tuberculosis: the endTB trial. Clin Trials. 2017;14(1):17–28. https://doi.org/10.1177/1740774516665090.
Phillips PPJ, Dooley KE, Gillespie SH, Heinrich N, Stout JE, Nahid P, et al. A new trial design to accelerate tuberculosis drug development: the phase IIC selection trial with extended posttreatment follow-up (STEP). BMC Med. 2016;14(1):1–11. https://doi.org/10.1186/s12916-016-0597-3.
Hatherill M, White RG, Hawn TR. Clinical development of new TB vaccines: recent advances and next steps. Front Microbiol. 2020;10(January):1–12. https://doi.org/10.3389/fmicb.2019.03154.
Sester M, van Crevel R, van Leth F, Lange C. Numbers needed to treat to prevent tuberculosis. Eur Respir J. 2015;46(6):1836–8. https://doi.org/10.1183/13993003.01047-2015.
Chen RY, Via LE, Dodd LE, et al. Using biomarkers to predict TB treatment duration (Predict TB): a prospective, randomized, noninferiority, treatment shortening clinical trial. Gates Open Res. 2017;1:9. https://doi.org/10.12688/gatesopenres.12750.1.
Walzl G, McNerney R, du Plessis N, Bates M, McHugh TD, Chegou NN, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis. 2018;18(7):e199–210. https://doi.org/10.1016/S1473-3099(18)30111-7.
Swindells S, Andrade-Villanueva J-F, Richmond GJ, Rizzardini G, Baumgarten A, Masiá M, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020;382(12):1112–23. https://doi.org/10.1056/NEJMoa1904398.
Orkin C, Arasteh K, Górgolas Hernández-Mora M, Pokrovsky V, Overton ET, Girard PM, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020;382(12):1124–35. https://doi.org/10.1056/NEJMoa1909512.
Swindells S, Siccardi M, Barrett SE, Olsen DB, Grobler JA, Podany AT, et al. Long-acting formulations for the treatment of latent tuberculous infection: opportunities and challenges. Int J Tuberc Lung Dis. 2018;22(2):125–32. https://doi.org/10.5588/ijtld.17.0486.
Rajoli RKR, Podany AT, Moss DM, Swindells S, Flexner C, Owen A, et al. Modelling the long-acting administration of anti-tuberculosis agents using PBPK: a proof of concept study. Int J Tuberc Lung Dis. 2018;22(8):937–44. https://doi.org/10.5588/ijtld.17.0515.
Kaushik A, Ammerman NC, Tyagi S, Saini V, Vervoort I, Lachau-Durand S, et al. Activity of a long-acting injectable bedaquiline formulation in a paucibacillary mouse model of latent tuberculosis infection. Antimicrob Agents Chemother. 2019;63(4):1–10. https://doi.org/10.1128/AAC.00007-19.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Elisa Ignatius is supported by T32 GM066691-17. Susan Swindells reports research grants to her institution from ViiV Healthcare and the National Institutes of Health.
Human and Animal Rights and Informed Consent
This article references studies with human subjects performed by Susan Swindells. The studies were approved by the relevant Institutional Review Boards/Ethics Committees, and all subjects gave written informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Co-infections and Comorbidity
Rights and permissions
About this article
Cite this article
Ignatius, E.H., Swindells, S. Are We There Yet? Short-Course Regimens in TB and HIV: From Prevention to Treatment of Latent to XDR TB. Curr HIV/AIDS Rep 17, 589–600 (2020). https://doi.org/10.1007/s11904-020-00529-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-020-00529-8