Abstract
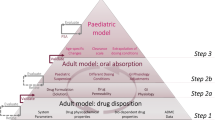
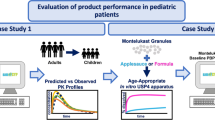
The importance of physiologically based pharmacokinetic (PBPK) model refinement with data acquired in adults using a pediatric formulation under age-relevant dosing conditions in order to extrapolate drug exposure to infants was recently demonstrated for paracetamol. In the present investigation, the aim was to evaluate the importance of similar PBPK model refinement for a low-solubility weak acid, ibuprofen, to simulate exposure across pediatric populations, i.e., infants, young children, and schoolchildren. After developing and evaluating adult disposition and oral absorption models for the aqueous suspension of ibuprofen, ibuprofen performance was extrapolated to pediatrics simulating exposure as a function of different prandial and dosing conditions: fasted conditions, reference-meal fed conditions (solid-liquid meal), and infant-formula fed conditions (homogeneous liquid). Successful predictions were achieved when employing the refined model for fasted state conditions or for fed state conditions relevant to specific age groups, i.e., infant formula for infants and reference meal for children. The present study suggested that ibuprofen performance was primarily guided by gastric emptying and showed sensitivity towards formulation characteristics and pH changes in the small intestine. Better understanding of luminal conditions in pediatrics and age-dependent ibuprofen post-absorptive processes could improve modeling confidence for ibuprofen, as well as other drugs with similar characteristics.






Similar content being viewed by others
References
Food and Drug Administration (FDA). Assessing the effects of food on drugs in INDs and NDAs-clinical pharmacology considerations guidance for industry. Guid Doc [Internet]. 2019. Available from https://www.fda.gov/media/121313/download. Accessed 24/10/2020.
European Medicines Agency (EMA). Guideline on the investigation of drug interactions. Guid Doc [Internet]. 2012;44:1–59. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf. Accessed 24/10/2020.
Guimarães M, Statelova M, Holm R, Reppas C, Symilllides M, Vertzoni M, Fotaki N. Biopharmaceutical considerations in paediatrics with a view to the evaluation of orally administered drug products – a PEARRL review. J Pharm Pharmacol. 2019;71(4):603–42.
Statelova M, Goumas K, Fotaki N, Holm R, Symillides M, Reppas C, Vertzoni M. On the design of food effect studies in adults for extrapolating oral drug absorption data to infants: an exploratory study highlighting the importance of infant food. AAPS J. 2020;22(6):1–11.
World Health Organization (WHO). Paediatric age categories to be used in differentiating between listing on a model essential medicines list for children. Position Pap. 2007;1–5.
Batchelor H. Influence of food on paediatric gastrointestinal drug absorption following oral administration: a review. Children. 2015;2(2):244–71.
Kostewicz ES, Aarons L, Bergstrand M, Bolger MB, Galetin A, Hatley O, et al. PBPK models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57(1):300–21.
Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50:S41–67.
Villiger A, Stillhart C, Parrott N, Kuentz M. Using physiologically based pharmacokinetic (PBPK) modelling to gain insights into the effect of physiological factors on oral absorption in paediatric populations. AAPS J. 2016;18(4):933–47.
Verscheijden LFM, Koenderink JB, Johnson TN, De Wildt SN, Russel FGM. Physiologically-based pharmacokinetic models for children: starting to reach maturation? Pharmacol Ther. 2020;10.
Abduljalil K, Pan X, Pansari A, Jamei M, Johnson TN. Preterm physiologically based pharmacokinetic model. Part II: applications of the model to predict drug pharmacokinetics in the preterm population. Clin Pharmacokinet. 2020;59(4):501–18.
Balbas-martinez V, Michelet R, Edginton AN, Meesters K, Trocóniz IF, Vermeulen A. Physiologically-based pharmacokinetic model for ciprofloxacin in children with complicated urinary tract infection. Eur J Pharm Sci. 2018; (128):171–9.
Rasool MF, Khalil F, Laaer S. A physiologically based pharmacokinetic drug-disease model to predict carvedilol exposure in adult and paediatric heart failure patients by incorporating pathophysiological changes in hepatic and renal blood flows. Clin Pharmacokinet. 2015;54(9):943–62.
Samant TS, Lukacova V, Schmidt S. Development and qualification of physiologically based pharmacokinetic models for drugs with atypical distribution behavior: a desipramine case study. CPT Pharmacometrics Syst Pharmacol. 2017;6(5):315–21.
Willmann S, Thelen K, Kubitza D, Lensing AWA, Frede M, Coboeken K, et al. Pharmacokinetics of rivaroxaban in children using physiologically based and population pharmacokinetic modelling : an EINSTEIN-Jr phase I study. Thromb J. 2018;16(32):1–12.
Cristofoletti R, Charoo NA, Dressman JB. Exploratory investigation of the limiting steps of oral absorption of fluconazole and ketoconazole in children using an in silico pediatric absorption model. J Pharm Sci. 2016;105(9):2794–803.
Johnson TN, Bonner JJ, Tucker GT, Turner DB, Jamei M. Development and applications of a physiologically-based model of paediatric oral drug absorption. Eur J Pharm Sci. 2018;115(June 2017):57–67.
Kohlmann P, Stillhart C, Kuentz M, Parrott N. Investigating oral absorption of carbamazepine in pediatric populations. AAPS J. 2017;19(6):1864–77.
Statelova M, Holm R, Fotaki N, Reppas C, Vertzoni M. Successful extrapolation of paracetamol exposure from adults to infants after oral administration of a paediatric aqueous suspension is highly dependent on the study dosing conditions. AAPS J. 2020;22(126):1–17.
Tsamandouras N, Rostami-Hodjegan A, Aarons L. Combining the “bottom up” and “top down” approaches in pharmacokinetic modelling: fitting PBPK models to observed clinical data. Br J Clin Pharmacol. 2015;79(1):48–55.
Food and Drug Administration (FDA). Guidance for industry food-effect bioavailability and fed bioequivalence studies. Guid Doc [Internet] 2002. Available from: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070241.pdf. Accessed 24/10/2020.
Potthast H, Dressman JB, Junginger HE, Midha KK, Oeser H, Shah VP, et al. Biowaiver monographs for immediate release solid oral dosage forms: ibuprofen. J Pharm Sci. 2005;94(10):2121–31.
Pavliv L, Voss B, Rock A. Pharmacokinetics, safety, and tolerability of a rapid infusion of i.v. ibuprofen in healthy adults. Am J Heal Pharm. 2011;68(1):47–51.
Chassard D, Geneteau A, Gualano V, Brault M. Bioequivalence study of two ibuprofen formulations administered intravenously in healthy male volunteers. Clin Drug Investig. 2004;24(12):739–47.
Martin W, Koselowske G, Töberich H, Kerkmann T, Mangold B, Augustin J. Pharmacokinetics and absolute bioavailability of ibuprofen after oral administration of ibuprofen lysine in man. Biopharm Drug Dispos. 1990;11(3):265–78.
Atkinson HC, Stanescu I, Frampton C, Salem II, Beasley CPH, Robson R. Pharmacokinetics and bioavailability of a fixed-dose combination of ibuprofen and paracetamol after intravenous and oral administration. Clin Drug Investig. 2015;35(10):625–32.
Khalil SN, Hahn BJ, Chumpitazi CE, Rock AD, Kaelin BA, Macias CG. A multicenter, randomized, open-label, active-comparator trial to determine the efficacy, safety, and pharmacokinetics of intravenous ibuprofen for treatment of fever in hospitalized pediatric patients Samia. BMC Pediatr. 2017;17(42):1–11.
Kokki H, Kumpulainen E, Lehtonen M, Laisalmi M, Heikkinen M, Savolainen J, et al. Cerebrospinal fluid distribution of ibuprofen after intravenous administration in children. Pediatrics. 2007;120(4):e1002 LP-e1008.
Brown RD, Wilson JT, Kearns GL, Eichler VF, Johnson VA, Bertrand KM. Single-dose pharmacokinetics of ibuprofen and acetaminophen in febrile children. J Clin Pharmacol. 1992;32:232–41.
Kelley MT, Walson PD, Edge JH, Cox S, Mortensen ME. Pharmacokinetics and pharmacodynamics of ibuprofen enantiomers and acetaminophen in febrile children. Clin Pharmacol Ther. 1992;52(2):181–9.
Walson PD, Galletta G, Pharm D, Braden NT, Alexander L, Columbus BS. Ibuprofen, acetaminophen, and placebo treatment of febrile children. Clin Pharmacol Ther. 1989;46(1):9–17.
Rey E, Pariente-Khayat A, Gouyet L, Vauzelle-Kervroedan F, Pons G, D’Athis P, et al. Stereoselective disposition if ibuprofen enantiomers in infants. Br J Clin Pharmacol. 1994;38:373–5.
Davies NM. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin Pharmacokinet. 1998;34(2):101–54.
Aarons L, Grennan DM, Siddiqui M. The binding of ibuprofen to plasma proteins. Eur J Clin Pharmacol. 1983;25(6):815–8.
Smith DE, Paliwal JK, Cox SR, Berardi RR, Dunn-Kucharski VA, Elta GH. The effect of competitive and non-linear plasma protein binding on the stereoselective disposition and metabolic inversion of ibuprofen in healthy subjects. Biopharm Drug Dispos. 1994;15(7):545–61.
Wagner JG, Albert KS, Szpunar GJ, Lockwood GF. Pharmacokinetics of ibuprofen in man IV: absorption and disposition. J Pharmacokinet Biopharm. 1984;12(4):381–99.
Somani AA, Thelen K, Zheng S, Trame MN, Coboeken K, Meyer M, et al. Evaluation of changes in oral drug absorption in preterm and term neonates for Biopharmaceutics Classification System (BCS) class i and II compounds. Br J Clin Pharmacol. 2016;81(1):137–47.
Barzilay B, Youngster I, Batash D, Keidar R, Baram S, Goldman M, et al. Pharmacokinetics of oral ibuprofen for patent ductus arteriosus closure in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2012;97(2):1–5.
Pharmacopoiea E. Ibuprofenum. Ph Eur. 2008;7(1):2225–7.
Avdeef A, Berger CM, Brownell C. pH-metric solubility. 2: correlation between the acid-base titration and the saturation shake-flask solubility-pH methods. Pharm Res. 2000;17(1):85–9.
Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man - fact or myth. Pharm Res. 1997;14(6):763–6.
Sun D, Lennernas H, Welage LS, Barnett JL, Landowski CP, Foster D, et al. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharm Res. 2002;19(10):1400–16.
Lu ATK, Frisella ME, Johnson KC. Dissolution modeling: factors affecting the dissolution rates of polydisperse powders. Pharm Res. 1993;10(9):1308–14.
Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: an examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos. 1999;27(11):1350–9.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, Neutrals and Zwitterions. J Pharm Sci. 2005;95(6):1238–57.
Lukacova V, Parrott NJ, Lave T, Fraczkiewicz G, Bolger MB. Role of fraction unbound in plasma in calculation of tissue:plasmapartition coefficients. In: AAPS National meeting, Atlanta, November 15–20. 2008.
Boase S, Miners JO. In vitro-in vivo correlations for drugs eliminated by glucuronidation: investigations with the model substrate zidovudine. Br J Clin Pharmacol. 2002;54(5):493–503.
Ladumor MK, Bhatt DK, Gaedigk A, Sharma S, Thakur A, Pearce RE, et al. Ontogeny of hepatic sulfotransferases and prediction of age-dependent fractional contribution of sulfation in acetaminophen metabolism. Drug Metab Dispos. 2019;47(8):818–31.
SimulationsPlus Inc. GastroPlus Manual. 2020.
T’jollyn H, Vermeulen A, Van Bocxlaer J. PBPK and its virtual populations: the impact of physiology on pediatric pharmacokinetic predictions of tramadol. AAPS J. 2019;21(1):1–12.
Mithani SD, Bakatselou V, TenHoor CN, Dressman JB. Estimation of the increase in solubility of drugs as function of bile salt concentration. Pharm Res. 1995;13(1):163–7.
Markopoulos C, Andreas CJ, Vertzoni M, Dressman J, Reppas C. In-vitro simulation of luminal conditions for evaluation of performance of oral drug products: choosing the appropriate test media. Eur J Pharm Biopharm. 2015;93:173–82.
Vertzoni M, Pastelli E, Psachoulias D, Kalantzi L, Reppas C. Estimation of intragastric solubility of drugs: in what medium? Pharm Res. 2007;24(5):909–17.
Pentafragka C, Vertzoni M, Symillides M, Goumas K, Reppas C. Disposition of two highly permeable drugs in the upper gastrointestinal lumen of healthy adults after a standard high-calorie, high-fat meal. Eur J Pharm Sci. 2020;149(105351):1–9.
Sanaka M, Kuyama Y, Shimomura Y, Saitoh M, Hattori K. New mathematical model for accurate description of absorption kinetics of Paracetamol given orally with a high calorie liquid meal. Int J Clin Pharmacol Ther. 2002;40(11):499–506.
United States Department of Health and Human Services. Centers for disease control and prevention. National center for health statistics. National health and nutrition examination survey III, 1988–1994: Series II, No. 3A. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2006-01-18. https://doi.org/10.3886/ICPSR04010.v1
Johnson TN, Rostami-hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates. Infants and Children. 2006;45(9):931–56.
Haddad S, Restieri C, Krishnan K. Characterization of age-related changes in body weight and organ weights from birth to adolescence in humans. J Toxicol Environ Heal - Part A. 2001;64(6):453–64.
Cristofoletti R, Dressman JB. Use of physiologically based pharmacokinetic models coupled with pharmacodynamic models to assess the clinical relevance of current bioequivalence criteria for generic drug products containing ibuprofen. J Pharm Sci. 2014;103:3263–75.
Edginton, A.N.; Willmann, S. Physiology-based versus allometric scaling of clearance in children; an eliminating process based comparison. Paediatric Perinat. Drug Ther. 2006; 7:146–153.
Calvier EAM, Krekels EHJ, Johnson TN, Tibboel D, Knibbe CAJ. Scaling drug clearance from adults to the young children for drugs undergoing hepatic metabolism: a simulation study to search for the simplest scaling method. AAPS J. 2019;21(3):38.
Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45(10):1013–34.
Cumberland Pharmaceuticals. Clinical pharmacology review new drug application: 22348. Pediatric Research Equity Act [Internet]. 2015: 1-79. Available from: https://www.fda.gov/media/95406/download. Accessed 24/10/2020
European Food Safety Authority. Scientific opinion on dietary reference values for energy. EFSA J. 2013;11(1):1–112.
Bonner JJ, Vajjah P, Abduljalil K, Jamei M, Rostami-Hodjegan A, Tucker GT, et al. Does age affect gastric emptying time? A model-based meta-analysis of data from premature neonates through to adults. Biopharm Drug Dispos. 2015;36(4):245–57.
Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, Macintyre F, et al. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther. 1997;283(1):46 LP–58.
Mahmood I, Ahmad T, Mansoor N, Sharib SM. Prediction of clearance in neonates and infants (≤ 3 months of age) for drugs that are glucuronidated: a comparative study between allometric scaling and physiologically based pharmacokinetic modeling. J Clin Pharmacol. 2017;57(4):476–83.
Grimm M, Scholz E, Koziolek M, Kuhn JP, Weitschies W. Gastric water emptying under fed state clinical trial conditions is as fast as under fasted conditions gastric water emptying under fed state clinical trial conditions is as fast as under fasted conditions. Mol Pharm. 2017;14(12):4262–71.
Kwatra NS, Shalaby-Rana E, Andrich MP, Tsai J, Rice AL, Ghelani SJ, et al. Gastric emptying of milk in infants and children up to 5 years of age: normative data and influencing factors. Pediatr Radiol. 2020;50(5):689–97.
Loisios-konstantinidis I, Cristofoletti R, Fotaki N, Turner DB, Dressman J. Establishing virtual bioequivalence and clinically relevant specifications using in vitro biorelevant dissolution testing and physiologically-based population pharmacokinetic modeling. Case example: naproxen. Eur J Pharm Sci. 2020;143:105170.
Lockwood GF, Albert KS, Gillespie WR, Bole GG, Harkcom TM, Szpunar GJ, et al. Pharmacokinetics ibuprofen in man. I. Free and total area/dose relationships. Clin Pharmacol Ther. 1983;34(1):97–103.
Acknowledgments
The authors would like to thank biorelevant.com for providing the SIF powder for the preparation of biorelevant media and to express their gratitude to SimulationsPlus Inc., for providing access to GastroPlus™ 9.7.
Funding
This work has received funding from the Horizon 2020 Marie Skłodowska-Curie Innovative Training Networks program under grant agreement no. 674909.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 497 kb)
Rights and permissions
About this article
Cite this article
Statelova, M., Holm, R., Fotaki, N. et al. Factors Affecting Successful Extrapolation of Ibuprofen Exposure from Adults to Pediatric Populations After Oral Administration of a Pediatric Aqueous Suspension. AAPS J 22, 146 (2020). https://doi.org/10.1208/s12248-020-00522-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-00522-4