Abstract
While characteristics, sources, and formation process of PM2.5 have been widely reported, only a few studies discussed PM2.5 in the Indian subcontinent, specifically in a densely populated city, like Patna. In this study, we investigated the chemical composition and source of PM2.5 in the central Indo-Gangetic Plain of India (Patna), during January–December, 2018. Principal component analysis (PCA) and air mass back trajectory were performed to mark the sources of PM2.5. The result showed that the average annual PM2.5 level in Patna (172 µg/m3) was about 4 times greater than the annual limit (40 µg/m3) set by national ambient air quality standard of India, indicating the dangerous level. Overall, the highest level of water-soluble inorganic ions (ΣWSIIs) was measured in winter, followed by autumn, and ranged from 2 to 60 µg/m3 (median 16 µg/m3) and 3 to 47 µg/m3 (median 14 µg/m3), respectively. NH4+, SO42−, and NO3− were the most abundant ions in PM2.5 and accounted for 38–51%, 21–28%, and 8–20% of ΣWSIIs, respectively. The PCA analysis indicated primary emission from mixed source (street dust, coal combustion, biomass burning, vehicular emission, and industrial emissions) and secondary formation from coal combustion are the essential source of air pollution in Patna. The air mass back trajectory analysis revealed that most of the air mass at Patna arrives mainly from westerly/northwesterly direction originating from Pakistan and Afghanistan in winter/autumn, while westerly/southwesterly winds originating from Arabian Sea and crossing through Bay of Bengal impacted air quality in summer/rainy season.
Similar content being viewed by others
1 Introduction
PM2.5, also known as fine particulate matter, is commonly characterized as the particles with an aerodynamic diameter ≤ 2.5 μm. PM2.5 is the main component of haze pollution worldwide [1, 2]. It is an important class of atmospheric pollutant and can cause various antagonistic effects on human well-being, reduces atmospheric visibility, and global climate [3, 4]. Rapid urbanization and industrialization have intensified PM2.5 pollution and environmental risk especially in developing countries such as China and India [5,6,7,8,9]. PM2.5 comprises primary as well as secondary particles. The primary particles are emitted legitimately from a source, for example, soil dust from fields, highway and construction sites, biological emissions from fires, and sea salt from the ocean. In contrast, the secondary particles are formed through complicated reactions of the atmospheric gases, such as SO2, NOx, and NH3 transmitted from different primary sources [2, 10].
The man-made PM2.5 is an amalgam of different constituents, such as metals, organic/elemental carbon compounds, nitrates, and sulfate. In these parts, water-soluble inorganic ions (WSIIs), for example, sulfate, nitrate, and ammonium are the significant constituents and accounted for more than 70% of the total PM2.5 mass [2, 6]. WSII in PM2.5 can significantly influence the hygroscopic behavior [6, 11] and acidic properties of PM2.5 [12]. Moreover, it plays an essential role in reduction in atmospheric visibility [1] and accelerating the formation of PM2.5 [13]. For example, sulfate and nitrate in PM2.5 can significantly reduce the light scattering potential of PM2.5 [14]. Along these lines, it is essential to examine the characteristics of PM2.5 with respect to WSIIs in order to understand the sources, behaviors, and development mechanism of PM2.5 [15]. Recently, PM2.5 has been received worldwide attention due to its immediate and roundabout effects on global air quality and climate visibility [1, 4], radiative balance [12, 16], and nutrient deposition [10]. Hence, to address these effects, chemical characterization, source/sinks apportionment, the formation mechanism of PM2.5 should be comprehended at the local, regional, and global level [17,18,19]. Extensive chemical monitoring combined with air mass trajectory models (such as concentration-based trajectory) and receptor models (such as principal component analysis) are some of the commonly used approaches for evaluating the location and types of PM2.5 pollution at the receptor site [20, 21].
Patna is one of the most densely populated cities of India with a total population of about 1.69 million, situated on the southern bank of river Ganges in the central Indo-Gangetic plain (IGP) of India. It is one of the highly populated and polluted river basins in the world. The climate of Patna is of humid subtropical type and is classified as “Cwa (monsoon-influenced humid subtropical climate)” as per Koppen’s climate classification. The hot season starts from early May to June, while early July to September is monsoon season in Patna. November to February is considered a chilly winter period, with the temperature reaching as low as 0 °C. The highest temperature during summer may reach up to 47 °C. Vehicular pollutions, industrial emissions, and construction activities are believed to be the primary sources of respirable suspended particulate matter in Patna [22]. In 2014, Patna was listed as the second most air polluted city in India, only after New Delhi by World Health Organization [23]. While the different constituents of PM2.5 and their sources of emission have been investigated worldwide [17, 24,25,26,27,28,29,30,31,32], such studies are yet limited in India, more specifically in the case of Patna. Recently, few studies reported the concentration of trace gases and carbonaceous component in particulate fractions from Patna [22, 33, 34]. However, the composition of PM2.5, especially WSIIs and their sources, remains deficient. This study aims to characterize the chemical composition and sources of PM2.5 pollutions on a seasonal basis. This paper is the extended version of a conference paper first presented at the International Conference on Air Quality, Health, and Atmosphere held in London, the UK during January 20–21, 2020 [34]. The outcome of the study will provide an insight into the characteristics, sources, and environmental effects of PM2.5 in Patna City of India.
2 Materials and methods
2.1 Study site and sampling
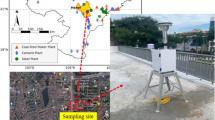
PM2.5 samples were collected at Patel Nagar, located in the central part of Patna City. The Patel Nagar was chosen as sampling site for measuring PM2.5 because it is a good representative of the urban environment, but free from direct source points such as high traffic area, Bus Park, or railway station. The Patel Nagar is located at 25.6216°N 85.0993°E (Fig. 1). PM2.5 samples were collected using high-volume sampler (HVS) (Model APM 550 M, Envirotech Pvt. Ltd.) installed on the rooftop of a residential building (about 10 m above the ground level). The sampling strategy was explained to the house owner, and his/her consent was obtained prior to installation of HVS on rooftop of the building. A total of 120 PM2.5 samples were collected on quartz fiber filter (QFF) (diameter 47 mm) during January to December 2018 representing four seasons, i.e., winter (December through February), summer (March through May), rainy (June through September), and autumn (October through November). The individual QFF was exposed for 3 days (72 h) with an average flow rate of 1 m3/h. Before sampling, the QFF was pre-baked at 350–400 °C for 6 h in an oven to avoid contamination. The mass of PM2.5 was analyzed gravimetrically. The initial (before exposed) and final (after exposed) weights of QFFs were taken at least three times at room temperature using a microelectronic balance with an accuracy of ± 1 µg. Additionally, field blank samples were collected to assess the possible cross-contamination during the sampling. The QFF filter was wrapped in aluminum foil, sealed in zipper plastic bags, and stored at − 20 °C in a refrigerator until chemical analysis.
2.2 Chemical analysis of PM2.5
A part of QFF was used for the analysis of WSIIs following a standard protocol discussed previously [34,35,36]. Briefly, a portion (2.8 cm2) of QFF was cut and extracted in a sonicator for 1 h using 50 mL of Milli-Q water (resistivity > 18.2 MΩ). The extracted samples were centrifuged and filtered using a syringe filter of 0.2 µm. The filtered sample was stored in a refrigerator until chemical analysis. The target cations (Ca2+, Mg2+, Na+, K+, NH4+) and anions (Cl−, SO42−, NO3−) components of PM2.5 were analyzed by ion chromatography (Metrohm make, Model 881 compact IC). The two different columns (metro SepA Supp 5–250 mm and metro Sep C 4–150 mm for cations and anions, respectively) were used to separate cations and anions. Seven blank filters were also tested for cation and anions content as part of QA/QC. The new filters were wrapped in aluminum foil and taken to sampling sites keeping in a plastic zipper bag. Later, they were returned with original samples and transported to the laboratory for chemical analysis. The concentration of cation and anions in blank samples varied from 0.04 to 0.64 µg/m3 and 0.05 to 0.19 µg/m3, respectively. The method detection limit (MDL) was estimated as three times a standard deviation plus mean of all blanks. In the case of non-detection of the species in the blank, MDL was determined as three times the standard deviation of the lowest spiked standard. The MDL ranged from 0.10 to 0.87 µg/m3 for anions and 0.11 to 1.90 µg/m3 for cations, respectively.
3 Results and discussion
3.1 Seasonal variation of PM2.5
The annual statistical summary of PM2.5, along with the past studies reported in other Indian cities, is given in Table 1. Meteorological data were obtained from the Indian Meteorological Department, Government of India (Table S1). In this study, the highest concentration of PM2.5 occurred in autumn and winter and ranged from 180 to 634 µg/m3 (median 350 µg/m3) and 52 to 368 µg/m3 (median 135 µg/m3), respectively (Fig. 2). Relatively, low concentration of PM2.5 was measured in summer and rainy seasons and ranged from 45 to 487 µg/m3 (median 112 µg/m3) and 34 to 214 µg/m3 (median 91 µg/m3), respectively. The highest concentration of PM2.5 in autumn is likely due to biomass burning and dispersion of pollutants due to the low boundary layer height (BLH) [37]. The post-monsoon biomass burning activities in the Indian states of Punjab and Haryana could be the possible reason for dramatic increase in PM2.5. It is estimated that about 70–80 million tons of rice stubble is burnt in an open field [38, 39]. Also, the meteorology, over the Indian subcontinent, mostly favors the transport of emissions eastward along the Himalayas [40]. A sudden peak of PM2.5 has been also reported previously during October–November [40,41,42,43]. A relatively low level of PM2.5 in summer and rainy seasons could be because of little wind and lower mixing height leading to better dispersion and deposition. The strong wind in summer and frequent rainfall in Rainy seasons could equally contribute to a low concentration of PM2.5. The annual average PM2.5 concentration measured in this study was about 4–5 times greater than the annual PM2.5 limit (40 µg/m3) set by the national ambient air quality standard (NAAQS) of India [44]. Moreover, this level is about 11 and 9 times higher than the PM2.5 limit of USEPA (15 µg/m3) [45] and the European Union (20 µg/m3), respectively [46]. This indicates that the PM2.5 pollutions at Patna are dangerous and need appropriate control measures to avoid human health risk. The PM2.5 concentration in this study was compared to past studies from India and abroad (Table 1). The annual average of PM2.5 in this study is consistent with past studies from Delhi [47] in India, Chengdu [48], and Beijing [49] in China. A highest monthly average PM2.5 concentration was measured in November (Fig. 2). Also, the winter PM2.5 concentrations in this study were higher than many urban areas in India (Table 1), such as Kanpur [43], Lucknow [50], and Agra [51] but several folds higher than Varanasi [52], Kolkata [53], and New Delhi [54].
3.2 Seasonal variation of WSIIs
In this study, the seasonal variation of WSIIs in PM2.5 is illustrated in Table S2 and Fig. 3. The sum of ΣWSIIs (Na+, K+, Ca2+, Mg2+, NH4+, Cl−, SO42−, and NO3−) accounted for about 11–30% of the total particle mass concentration. Overall, the highest level of ΣWSIIs was detected in winter, followed by autumn, and ranged from 2 to 60 µg/m3 (median 16 µg/m3) and 3 to 47 µg/m3 (median 14 µg/m3), respectively. The profile of the WSIIs depicted in Fig. 4 showed that NH4+, SO42−, and NO3− were the predominant ions in Patna and accounted for 38–51%, 21–28%, and 8–20% of ΣWSIIs, respectively. This finding is consistent with the previous study from Beijing, China [56]. The concentrations of NH4+ in PM2.5 in this study ranged from 0.6 to 16 µg/m3 (median 6.3 µg/m3), 0.3 to 21 µg/m3 (median 4.3 µg/m3) nd–14 µg/m3 (median 1.6 µg/m3), and 0.3 to 17 µg/m3 (median 7.3 µg/m3) for winter, summer, rainy, and autumn, respectively. The NH4+ concentration in PM2.5 in this study is comparable with past studies from western India (0.8–16.8 µg/m3) [57], Delhi (2.49–19.23 µg/m3) [58]. The elevated level of NH4+ in autumn and winter seasons could be possibly due to high relative humidity (RH) in the air, thereby favoring the conversion of NH3+ to NH4+ [59]. The application of fertilizer in the agricultural farm could also lead to a high level of NH4+. The high ambient temperature may also speed the volatilization of NH3+ [60, 61]. Furthermore, the death and decay of plants and other organic materials can significantly influence the concentration of NH4+ during wet conditions. It is opined that about half of the global emission of NH4+ comes from the Asia region [62]. The secondary pollutants, for instance, NO3− and SO42− were the second most abundant chemicals measured in PM2.5 after NH4+. The SO42− concentration ranged from 0.6 to 12 µg/m3 (median 3.73 µg/m3), 0.14 to 10.3 µg/m3 (median 2.98 µg/m3), 0.24 to 4.72 µg/m3 (median 0.72 µg/m3), and 0.66 to 5.66 µg/m3 (median 2.92 µg/m3) in winter, summer, rainy, and autumn, respectively. In this study, the SO42−concentration was much lower than those reported in Kanpur (17 µg/m3) [63], Delhi (7.11–19.01 µg/m3) [58], Andhra Pradesh (19.76 µg/m3) of India [64], however, comparable with other global studies (Table S3). The NO3− concentration in winter, summer, rainy and autumn ranged from non-detectable (nd)–19.3 µg/m3 (median 5.21 µg/m3), nd–11.7 µg/m3 (1.56 µg/m3), nd–2.63 µg/m3 (median 0.82 µg/m3), and 0.67–3.72 µg/m3 (median 1.19 µg/m3), respectively. This level of NO3− in this study is about 3–5 times lower than those reported in Delhi, India (2.60–18.94 µg/m3), and Kanpur, India (21 µg/m3) [58, 63], but, several folds higher than those reported in Mumbai, India (0.97 µg/m3), Pune (1.06 µg/m3), and Mangalore (0.16 µg/m3) [65,66,67]. These secondary pollutants are believed to release in the atmosphere from the oxides of sulfur and nitrogen through chemical reaction [68]. The SO42− concentration was more dominant in PM2.5 than NO3−. This finding is consistent with the previous study [64].
3.3 Aerosol acidity in the atmosphere
The cation and anion concentrations in ambient air are significantly influenced by natural and anthropogenic sources, such as marine saltwater [69]. In this study, the non-sea salt (nss-Ca2+, nss-K+, and nss-SO42−) was estimated for four different seasons to check the aerosol acidity. The non-sea salt result was obtained using equation [1] given below. The reference seawater value (K+/Na+: 0.0370), (Ca2+/Na+:0.0382), and (SO42−/Na+: 0.251) was obtained from the literature [70].
Result showed greatest contribution of non-sea salt ions (Ca2+ (52.7 ± 92.9 meq/m3), nss-K+ (129.9 ± 225.3 meq/m3), and nss-SO42− (29.9 ± 50.4 meq/m3)) in winter nss—followed by rainy season [nss-Ca2+ (10.8 ± 32.5 meq/m3), nss-K+ (17.9 ± 38.8 meq/m3), and nss-SO42− (22.2 ± 53.33 meq/m3)] (Fig. S1). The non-sea salt ions during summer and autumn seasons were nss-Ca2+ (1.7 ± 3.1 meq/m3), nss-K+ (6.2 ± 10.3 meq/m3), and nss-SO42− (3.4 ± 7.0 meq/m3) and nss-Ca2+ (7.8 ± 13.3 meq/m3), nss-K+ (11.2 ± 10.9 meq/m3), and nss-SO42− (4.5 ± 5.7 meq/m3), respectively.
The presence of anion species (SO42− and NO3−) in aerosol can cause acidity in atmosphere. These secondary pollutants are the oxidation product of SO2 and NOx, and their acidity property is neutralized by cation species (Ca2+, Mg2+, K+, and NH4+) [71]. The equivalent concentration of water-soluble cation and anions is a significant indicator to reveal the acidity of the environment. In this study, the equivalent concentration of selected major cations (K+, Ca2+, and NH4+) and anions (SO42−, NO3−) was estimated by dividing the level of ion chosen with their respective equivalent weight. A regression analysis was conducted to check the significant linkage between the acidity and alkalinity ions. The cation and anions showed considerable correlation to each other in winter (r2= 0.79) and rainy seasons (r2= 0.64) (Fig. 5). This indicates the dominance of acidic component in winter and rainy seasons. In this study, the dominance of acidic component in PM2.5 could be due to relatively high SO4 and NO3 content in winter than other season leading to formation of organosulfate and nitrate (Table S2). In rainy, the formation of particulate sulfate and nitrate is intensified by atmospheric oxidant and water vapor (NFRAQS, 1998). NOx and SOx must be converted to nitric acid and sulfuric acid prior to reaction with other chemicals to form PM2.5. Ammonia plays a key role in neutralizing the acidity of aerosols in atmosphere [72]. In this study, the proportion of NH4 + was less in rainy and winter seasons to neutralize the acidity of PM2.5. Likewise, cations and anions were positively correlated with each other in summer (r2= 0.48). However, these ions were poorly connected in autumn (r2= 0.08). A substantial increase in contribution of alkaline dust in summer can result in significant decrease in acidity of PM2.5 [73].
4 Source assessment
Three different independent approaches (e.g., correlation coefficient, principal component analysis, and air mass back trajectory analysis) were tested using the PM2.5 data set in order to apportion the sources of PM2.5 pollution in Patna, India. The results of these tests are summarized below.
4.1 Pearson’s correlation analysis
The interrelationship among WSIIs constituents of PM2.5 was tested using the SPSS software 21 version. The strongly correlated WSIIs suggest identical source of emission, while poorly correlated WSIIs specify different sources.
The result showed that the majority of WSIIs was positively linked to each other. The K+ and SO42− were positively linked to each other (R = 0.549, P < 0.05) indicating similar anthropogenic source, possible from biomass burning (Table S4). Similarly, NH4+ and K+ showed significant correlation (R = 0.484, P < 0.05), indicating emission from wood-burning activities [74, 75] because K+ mainly emitted from wood-burning due to cooking or heating purposes. The Ca2+ and Mg2+ were positively correlated (R = 0.586, P < 0.05) suggesting their emission from natural soil dust A moderately correlated NO3− and SO42− (R = 0.420, P < 0.05) indicated their similar sources from coal combustion. The ratios of NO3−/SO42− are commonly used to indicate the sources of these two ionic species. NO3−/SO42− ratios > 1 indicate the greater contribution of NO3− through mobile or vehicular emission, while NO3−/SO42− ratio < 1 suggests ample contribution of SO42− from industrial activity [76]. In this study, the NO3−/SO42− ratios ranged from 0 to 7.5 (1.2 ± 1.8) in rainy season, indicating mixture of mobile source and industrial emission. The NO3−/SO42− ratios in summer ranged from 0 to 70.5 (4.3 ± 15.6), autumn 0 to 4.2 (0.7 ± 1.4), and winter 0.3 to 1.33 (0.82 ± 0.42), respectively.
4.2 Principal component analysis (PCA)
The principal component analysis (PCA) was performed on the WSIIs data set separately in each season to investigate the sources of WSIIs (Table 2). In winter, a total of four principal components (PC) were extracted with an eigenvalue greater than 1 and a cumulative variance of 74.99%. PC 1 accounted for 30.44% of the variation in WSIIs data was positively loaded with Na+, K+, Mg2+, and NH4+. K+ is also an indicator of biomass burning. PC 2 comprised 16.92% of the variance in data and was highly loaded with and Ca2+ and NO3−. High loadings of Ca2+, Mg 2+, and Na+ are identified as the dust source and can generally originate from multiple sources such as tire and brake lining wear, automobile exhaust, surface weathering of the street, garden soil, and debris of leaf [77]. PC 3 contained 14.67% of the variation in WSIIs data and was positively loaded with SO42− (0.638). The SO42− is formed via the oxidation of SO2, which is produced primarily by coal combustion and some biomass burning [78], while NO3− generally originates from the oxidation of NOx, which is derived primarily from vehicle exhaust [79]. PC 4 accounted for 12.94% of data variance and was negatively loaded with Cl−. Hence, this factor is a mixed source.
In summer, three PC were extracted with an eigenvalue greater than 1, with a cumulative variance of 71.04%. PC 1 accounted for 36.61% and was highly loaded with Na+, SO42−, NH4+, and Cl−. A high contribution of SO42−, NH4+, Cl−, and Na+ is identified as secondary sulfate. High SO42− and NH4+ are also released from an industrial source, such as brick kiln [22, 80, 81]. PC 2 explored 19.97% of the total variance in data and was positively loaded with Cl− and NO3−. Cl− is believed to be associated with multiple sources such as coal burning, biomass burning, and sea salts [82]. PC 3 explained 14.42% data variation and was highly loaded with Ca2+ and Mg2+. In rainy season, a total of three PC were extracted with an eigenvalue greater than 1 with the cumulative variance of 68.88%. PC 1 contained 34.07% of the total variance and was loaded with Ca2+, Mg2+ Cl−, and NO3−. The dominance of Cl− and NO3− is an indication of secondary formation. The reaction of NOx with hydroxyl radicals leads to the formation of nitrate. PC 2 accounted for 21.39% of data variance with positive loading on Cl−, SO42−, and NH4+. PC 3 explained 13.42% of data variance and was highly loaded with K+. In autumn, only two main PC were extracted with an eigenvalue more significant than one and a cumulative variance of 82.45%. PC 1 accounted for 42.371% of data variation and positively linked with Na+, Mg2+, Cl−, and NO3−. PC 2 comprised 39.67% of data variation and was highly loaded with K+, Ca2+, SO42−, and NH4+.
In summary, the PCA analysis indicated primary emission from mixed source such as street dust, coal combustion, biomass burning, vehicular emission, and industrial emissions such as Kiln and secondary formation from coal combustion are the primary source of air pollution in Patna.
4.3 Back trajectory analysis
Back trajectory analysis is an essential tool in atmospheric science to check the sources of air mass movement with pollutants [51]. In this study, five-day isentropic air mass back trajectories were computed to examine the influence of air mass originating from a nearby or remote area on aerosols composition. HYSPLIT model recommended by US National Oceanic and Atmospheric Administration Air Resources Laboratory (NOAA ARL) (version 4) was utilized [83] with a global data analysis system (GDAS) (0.5° × 0.5°) archived data set. We chose vertical velocity at the height of 500 m above the ground level, and the start time was 5.00 UTC for each sampling day. Figure 6 shows the origin of air masses influencing the current study site for winter, summer, rainy, and autumn seasons based on backward air mass trajectory. Generally, the air mass movement was westerly or northwesterly direction originating from Pakistan and Afghanistan in winter/autumn and changes gradually to westerly/southwesterly direction during summer/rainy seasons. In summer and rainy, the air mass mostly circulates over southern India originating from Arabian sea and eastern India crossing through Bay of Bengal. Interestingly, none of the seasons showed air mass originating from northeastern region. This is because of high mountain in northeastern region thereby restricting the movement of air masses.
5 Conclusion
In this study, chemical characterization, sources, and the seasonal variation of PM2.5 were investigated to mark the air quality of Patna, India. The average PM2.5 concentrations exceeded the annual standard limit set by NAAQS of India, USEPA, and European Union. This indicates dangerous level of PM2.5 pollutions at Patna and needs appropriate control measures to avoid human health risk. The highest concentration of ΣWSIIs was detected in winter, followed by autumn seasons. NH4+, SO42−, and NO3− were the most abundant constituents in PM2.5 and accounted for 38–51%, 21–28%, and 8–20% of ΣWSIIs, respectively. The non-sea salt estimation showed dominance of acidic component in winter and rainy seasons. The large part of air quality in Patna is affected by local sources (such as street dust, coal combustion, biomass burning; vehicular emission and industrial emission such as Kiln, and secondary formation) as well as long-range transport from the non-source site (westerly/northwesterly in winter/autumn and southwesterly/Bay of Bengal in summer/rainy).
References
Yuan CS et al (2006) Correlation of atmospheric visibility with chemical composition of Kaohsiung aerosols. Atmos Res 82:663–679
Dai W et al (2013) Chemical composition and source identification of PM2.5 in the suburb of Shenzhen, China. Atmos Res 122:391–400
Lemieux PM, Lutes CC, Santoianni DA (2004) Emissions of organic air toxics from open burning: a comprehensive review. Prog Energy Combust Sci 30(1):1–32
Liu Z et al (2017) Size-resolved aerosol water-soluble ions during the summer and winter seasons in Beijing: formation mechanisms of secondary inorganic aerosols. Chemosphere 183:119–131
Li XR et al (2013) Characterization of the size-segregated water-soluble inorganic ions in the Jing-Jin-Ji urban agglomeration: spatial/temporal variability, size distribution and sources. Atmos Environ 77:250–259
He QS et al (2017) Characterization and source analysis of water-soluble inorganic ionic species in PM2.5 in Taiyuan city, China. Atmos Res 184:48–55
Prasad AK, Singh RP, Kafatos M (2006) Influence of coal based thermal power plants on aerosol optical properties in the Indo-Gangetic basin. Geophys Res Lett 33(5):07808
van Donkelaar D et al (2010) Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: development and application. Environ Health Perspect 118:847–855
Pandey A, Venkataraman C (2014) Estimating emissions from the Indian transport sector with on-road fleet composition and traffic volume. Atmos Environ 98:123–133
Xiao HW et al (2016) Atmospheric aerosol compositions over the South China Sea: temporal/variability and source apportionment. Atmos Chem Phys Discuss 17:3199–3214
Shen ZX et al (2009) Ionic composition of TSP and PM2.5 during dust storms and air pollution episodes at Xi’an. China Atmos Environ 43:2911–2918
Gao X et al (2011) Semi-continuous measurement of water-soluble ions in PM2.5 in Jinan, China: temporal variations and source apportionments. Atmos Environ 45:6048–6056
Zhou H et al (2018) Stoichiometry of water-soluble ions in PM2.5: application in source apportionment for a typical industrial city in semi-arid region, Northwest China. Atmos Res 204:149–160
Chen J et al (2014) Impact of relative humidity and water soluble constituents of PM2.5 on visibility impairment in Beijing, China. Aerosol Air Qual Res 14:260–268
Wang HL et al (2015) Water-soluble ions in atmospheric aerosols measured in five sites in the Yangtze River Delta, China: size-fractionated, seasonal variations and sources. Atmos Environ 123:370–379
Contini D et al (2014) Source apportionment of size-segregated atmospheric particles based on the major water-soluble components in Lecce (Italy). Sci Total Environ 472:248–261
Putaud J et al (2004) A European Aerosol Phenomenology-2: chemical characteristics of particulate matter at Kerbside, Urban, Rural and Background Sites in Europe. Atmos Environ 38:2579–2595
Querol X et al (2004) Speciation and origin of PM10 and PM2.5 in selected European cities. Atmos Environ 38:6547–6555
Solomon PA, Sioutas C (2008) Continuous and semicontinuous monitoring techniques for particulate matter mass and chemical components: a synthesis of findings from EPA’s Particulate Matter Supersites Program and related studies. J Air Waste Manag Assoc 58(2):164–195
Yang H et al (2016) Composition and sources of PM2.5 around the heating periods of 2013 and 2014 in Beijing: implications for efficient mitigation measures. Atmos Environ 124:378–386
Zhang YP et al (2017) Seasonal variation and potential source regions of PM2.5-bound PAHs in the megacity Beijing, China: impact of regional transport. Environ Pollut 231:329–338
Arif M et al (2018) Ambient black carbon, PM2. 5 and PM10 at Patna: influence of anthropogenic emissions and brick kilns. Sci Total Environ 624:1387–1400
World Health Organization (2014) Global status report on noncommunicable diseases 2014 (No. WHO/NMH/NVI/15.1). World Health Organization
Chan YC et al (1999) Source apportionment of PM2.5 and PM10 aerosols in Brisbane (Australia) by receptor modelling. Atmos Environ 33(19):3251–3268
Lee HS, Kang BW (2001) Chemical characteristics of principal PM2.5 species in Chongju, South Korea. Atmos Environ 35:739–746
Lin JJ (2002) Characterization of the major chemical species in PM2.5 in the Kaohsiung City, Taiwan. Atmos Environ 36:1911–1920
Ho KF et al (2003) Characterization of chemical species in PM2.5 and PM10 aerosols in Hong Kong. Atmos Environ 37:31–39
Hueglin C et al (2005) Chemical characterisation of PM2.5, PM10 and coarse particles at urban, near-city and rural sites in Switzerland. Atmos Environ 39:637–651
Lonati G et al (2005) Major chemical components of PM2.5 in Milan (Italy). Atmos Environ 39:1925–1934
Kumar R, Srivastava SS, Kumari KM (2007) Characteristics of aerosols over suburban and urban site of semiarid region in India: seasonal and spatial variations. Aerosol Air Qual Res 7(4):531–549
Ali MA, Assiri M, Dambul R (2017) Seasonal aerosol optical depth (AOD) variability using satellite data and its comparison over Saudi Arabia for the period 2002–2013. Aerosol Air Qual Res 17(5):1267–1280
Mallik C et al (2014) Variability of SO2, CO, and light hydrocarbons over a megacity in Eastern India: effects of emissions and transport. Environ Sci Pollut Res 21(14):8692–8706
Tiwari S et al (2016) Observations of ambient trace gas and PM10 concentrations at Patna, Central Ganga Basin during 2013–2014: the influence of meteorological variables on atmospheric pollutants. Atmos Res 180:138–149
Devi NL, Kumar A (2020) Long term Monitoring and Assessment of Atmospheric Aerosols in Indo-Gangetic Region of India. In: Conference proceedings, London, UK, 20–21 Jan 2020. Part XI
Devi NL, Kumar A, Yadav IC (2020) PM10 and PM2.5 in Indo-Gangetic Plain (IGP) of India: chemical characterization, source analysis, andtransport pathways. Urban Clim Urban Clim 33:100663. https://doi.org/10.1016/j.uclim.2020.100663
USEPA (1999) Particulate matter (PM2.5) Speciation guidance, document, Final Draft, Edition 1, October 7, 1999, U.S. EPA document. https://www3.epa.gov/ttnamti1/files/ambient/pm25/spec/specpln2.pdf. Accessed May 2020
Guinot B et al (2006) Impact of vertical atmospheric structure on Beijing aerosol distribution. Atmos Environ 40(27):5167–5180
Badarinath KVS, Chand TK, Prasad VK (2006) Agriculture crop residue burning in the Indo-Gangetic Plains—a study using IRS-P6 AWiFS satellite data. Curr Sci 91:1085–1089
Gadde B et al (2009) Air pollutant emissions from rice straw open field burning in India, Thailand, and the Philippines. Environ Pollut 157:1554–1558
Kaskaoutis DG et al (2014) Effects of crop residue burning on aerosol properties, plume characteristics, and long, range transport over northern India. J Geophys Res 119:5424–5444
Badarinath KVS et al (2009) Black carbon aerosol mass concentration variation in urban and rural environments of India—a case study. Atmos Sci Lett 10(1):29–33
Awasthi A et al (2011) Study of size and mass distribution of particulate matter due to crop residue burning with seasonal variation in rural area of Punjab, India. J Environ Monit 13:1073–1081
Ram K et al (2012) Carbonaceous and secondary inorganic aerosols during wintertime fog and haze over urban sites in the Indo-Gangetic Plain. Aerosol Air Qual Res 12:359–370
CPCB (2020) Central Pollution Control Board Environment data, Air quality data. https://cpcb.nic.in/automatic-monitoring-data/
EPA (2020), the United States Environmental Protection Agency, Environmental topics Air Topics Pollution and air quality. http://www.epa.gov/air/criteria.html
Environment (2020) European Commission Environment, Air pollution. http://ec.europa.eu/environment/air/quality/standards.htm
Nagar PK et al (2017) Characterization of PM 25 in Delhi: role and impact of secondary aerosol, burning of biomass, and municipal solid waste and crustal matter. Environ Sci Pollut Res 24(32):25179–25189
Tao J et al (2013) Chemical composition of PM2. 5 in an urban environment in Chengdu, China: importance of springtime dust storms and biomass burning. Atmos Res 122:270–283
Zhao X et al (2009) Seasonal and diurnal variations of ambient PM2.5 concentrations in urban and rural environments in Beijing. Atmos Environ 43:2893–2900
Barman SC et al (2008) Ambient air quality of Lucknow City (India) during the Use of fireworks on Diwali Festival. Environ Monit Assess 137(1–3):495–504
Kumar R, Kumari KM (2015) Aerosols and trace gases characterization over Indo-Gangetic plain in a semiarid region. Urban Clim 12:11–20
Mukherjee S et al (2018) Seasonal variability in chemical composition and source apportionment of sub-micron aerosol over a high altitude site in the Western Ghats, India. Atmos Environ 180:79–92
Das R et al (2015) Trace element composition of PM2.5 and PM10 from Kolkata—a heavily polluted Indian metropolis. Atmos Pollut Res 6:742–750
Tiwari S et al (2012) Variations in the mass of the PM 10, PM 2.5 and PM 1 during the monsoon and the winter at New Delhi. Aerosol and Air Quality Research. 12(1):20–29
Rastogi N (2014) Chemical characteristics of PM2. 5 at a source region of biomass burning emissions: evidence for secondary aerosol formation. Environ Pollut 184:563–569
Duan FK et al (2006) Concentration and chemical characteristics of PM2.5 in Beijing, China: 2001–2002. Sci Total Environ 355(1–3):264–275
Sudheer AK, Rengarajan R (2015) Time-resolved inorganic chemical composition of fine aerosol and associated precursor gases over an urban environment in western India: gas-aerosol equilibrium characteristics. Atmos Environ 109:217–227
Sharma SK, Mandal TK (2017) Chemical composition of fine mode particulate matter (PM2. 5) in an urban area of Delhi, India, and its source apportionment. Urban Climate 21:106–122
Singh S, Kulshrestha UC (2012) Abundance and distribution of gaseous ammonia and particulate ammonium at Delhi, India. Biogeosciences 9:5023–5029
Bouwman AF et al (1997) A global high-resolution emission inventory for ammonia. Global Biogeochem Cy 11:561–587
Goebes MD, Strader R, Davidson C (2003) An ammonia emission inventory for fertilizer application in the United States. Atmos Environ 37:2539–2550
Agriculture research service (2020) Agriculture research service, Natural resource, and sustainable agriculture systems, soil, and air. http://www.ars.usda.gov/research/programs
Rai P et al (2016) Composition and source apportionment of PM1 at urban site Kanpur in India using PMF coupled with CBPF. Atmos Res 178:506–520
Bisht DS et al (2015) Aerosol characteristics at a rural station in southern peninsular India during CAIPEEX-IGOC: physical and chemical properties. Environ Sci Pollut Res 22(7):5293–5304
Kumar R, Elizabeth A, Gawane AG (2006) Air quality profile of inorganic ionic composition of fine aerosols at two sites in Mumbai City. Aerosol Sci Technol 40(7):477–489
Gawhane RD et al (2017) Seasonal variation of chemical composition and source apportionment of PM 2.5 in Pune, India. Environ Sci Pollut Res 24(26):21065–21072
Kalaiarasan G et al (2018) Source apportionment studies on particulate matter (PM10 and PM2. 5) in ambient air of urban Mangalore, India. J Environ Manage 217:815–824
Seinfeld J, Pandis S (1998) Atmospheric chemistry and physics. Wiley, New York
Xiao J et al (2017) Use of sea-sand and seawater in concrete construction: current status and future opportunities. Constr Build Mater 155:1101–1111
Millero FJ (2006) Chemical oceanography, 3rd edn. CRC Press, Boca Raton, p 496
Han R et al (2007) Seismic slip record in carbonate-bearing fault zones: an insight from high-velocity friction experiments on siderite gouge. Geology 35(12):1131–1134
Wu X, Deng J, Chen J, Hong Y, Xu L, Yin L, Du W, Hong Z, Dai N, Yuan CS (2017) Characteristics of water-soluble inorganic components and acidity of PM2.5 in a coastal city of China. Aerosol Air Qual Res 17(9):2152–2164
Rastogi N, Sarin MM (2005) Long-term characterization of ionic species in aerosols from urban and high-altitude sites in western India: role of mineral dust and anthropogenic sources. Atmos Environ 39:5541–5554
Draxler RR, Hess GD (1998) An overview of the HYSPLIT_4 modeling system for trajectories. Aust Meteorol Mag 47(4):295–308
Cheng Z et al (2013) Long-term trend of haze pollution and impact of particulate matter in the Yangtze River Delta, China. Environ Pollut 182:101–110
Arimoto R et al (1996) Atmospheric non-sea-salt sulfate, nitrate, and methanesulfonate over the China Sea. Journal of Geophysical Research: Atmospheres 101(D7):12601–12611
Rogge WF, Mazurek MA, Hildemann LM, Cass GR, Simoneit BRT (1993) Quantification of urban organic aerosols at a molecular-level-identification, abundance, and seasonal variation. Atmos. Environ. Part A-Gen. Top. 27:1309–1330
Wang Y, Zhuang GS, Zhang XY et al (2006) The ion chemistry, seasonal cycle, and sources of PM2.5 and TSP aerosol in Shanghai. Atmos Environ 40:2935–2952
Deng XL, Shi CE, Wu BW et al (2016) Characteristics of the water-soluble components of aerosol particles in Hefei, China. J. Environ. Sci. 42:32–40
Yu LD, Wang GF, Zhang RJ, Zhang LM, Song Y, Wu BB et al (2013) Characterization and source apportionment of PM2.5 in an urban environment in Beijing. Aerosol Air Qual. Res. 13:574–583
Yao L, Yang LX, Yuan Q, Yan C, Dong C, Meng CP et al (2016) Sources apportionment of PM2.5 in a background site in the North China plain. Sci Total Environ 541:590–598
Liu B, Li T, Yang J, Wu J, Wang J, Gao J, Bi X, Feng Y, Zhang Y, Yang H (2017) Source apportionment and a novel approach of estimating regional contributions to ambient PM2.5 in Haikou. China. Environ. Pollut. 223:334–345
Tiwari S et al (2009) Black carbon and chemical characteristics of PM10 and PM2.5 at an urban site of North India. J Atmos Chem 62(3):193–209
Acknowledgements
This work was supported to NLD by extra-mural research funded by The Science and Engineering Research Board, Department of Science and Technology (SERB-DST), Government of India (EMR/2016/000052).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, A., Yadav, I.C., Shukla, A. et al. Seasonal variation of PM2.5 in the central Indo-Gangetic Plain (Patna) of India: chemical characterization and source assessment. SN Appl. Sci. 2, 1366 (2020). https://doi.org/10.1007/s42452-020-3160-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-3160-y