Abstract
Background
Neoadjuvant chemotherapy (NAC) represents a promising alternative to pancreatic ductal adenocarcinoma (PDAC) planned resection, but the survival impact remains undefined. To assess the feasibility and survival outcomes of NAC with gemcitabine and S1 (GS) for PDAC planned resection by prospective study.
Methods
Patients with resectable or borderline resectable PDAC received 2 cycles of NAC-GS and were offered curative resection followed by gemcitabine adjuvant. The primary endpoint was 2-year overall survival (OS). Adverse events during NAC, radiological and tumor marker responses, resection rate, and surgical safety were evaluated as secondary endpoints (UMIN000004148).
Results
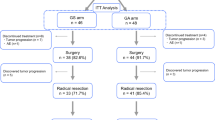
We enrolled 104 patients between 2010 and 2012, with 101 patients treated using NAC-GS as the full analysis set (FAS). Of the 101 patients, 88% received the planned 2 cycles of NAC. Grade 3 neutropenia was common (35%). Radiological partial response and decreased carbohydrate antigen 19-9 concentration (> 50% decrease) were noted in 13% and 41%, respectively. R0/1 resections with M0 were performed in 65 patients without surgical mortality. Of the 65 patients, 44 received planned gemcitabine adjuvant for 6 months as the on-protocol cohort. The primary endpoint for the 2-year OS rate was 55.9% in the FAS (n = 101) and 74.6% in the on-protocol cohort (n = 44).
Conclusions
NAC-GS was feasible and actively prolonged survival following PDAC planned resection. Randomized control trials are needed to further clarify the survival benefit of NAC-GS in addition to surgery followed by adjuvant therapy.




Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Saung MT, Zheng L. Current standards of chemotherapy for pancreatic cancer. Clin Ther. 2017;39:2125–34.
Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–80.
Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19:169–75.
Neoptolemos JP, Stocken DD, Freiss H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;351:1200–10.
Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–81.
Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–56.
Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248–57.
Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–24.
Friess H, Kleeff J, Silva JC, et al. The role of diagnostic laparoscopy in pancreatic and periampullary malignancies. J Am Coll Surg. 1998;186:675–82.
Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–82.
Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57.
Andriulli A, Festa V, Botteri E, et al. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol. 2012;19:1644–62.
D’Angelo F, Antolino L, Farcomeni A, et al. Neoadjuvant treatment in pancreatic cancer: evidence-based medicine? A systematic review and meta-analysis. Med Oncol. 2017;34:85.
Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31:1640–8.
Yanagimoto H, Ishii H, Nakai Y, et al. Improved survival with combined gemcitabine and S-1 for locally advanced pancreatic cancer: pooled analysis of three randomized studies. J Hepatobiliary Pancreat Sci. 2014;21:761–6.
Motoi F, Ishida K, Fujishima F, et al. Neoadjuvant chemotherapy with gemcitabine and S-1 for resectable and borderline pancreaticductal adenocarcinoma: results from a prospective multi-institutional phase 2 trial. Ann Surg Oncol. 2013;20:3794–801.
Gillen S, Schuster T, Zum Büschenfelde CM, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Pancreatic Adenocarcinoma Version 3. 2017. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed 19 Oct 2017.
Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v56–68.
Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–210.
Luberice K, Downs D, Sadowitz B, et al. Has survival improved following resection for pancreatic adenocarcinoma? Am J Surg. 2017;214:341–6.
Eshuis WJ, van der Gaag NA, Rauws EA, et al. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Ann Surg. 2010;252:840–9.
Michalski CW, Kleeff J, Wente MN, et al. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg. 2007;94:265–73.
Kang MJ, Jang JY, Kim SW. Surgical resection of pancreatic head cancer: what is the optimal extent of surgery? Cancer Lett. 2016;382:259–65.
Motoi F, Unno M, Takahashi H, et al. Influenceof preoperative anti-cancer therapy on resectability and perioperative outcomes in patients with pancreatic cancer: project study by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2014;21:148–58.
OʼReilly EM, Perelshteyn A, Jarnagin WR, et al. A single-arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg. 2014;260:142–8.
Ielpo B, Duran H, Diaz E, et al. Preoperative treatment with gemcitabine plus nab-paclitaxel is a safe and effective chemotherapy for pancreatic adenocarcinoma. Eur J Surg Oncol. 2016;42:1394–400.
Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. 2015;22:1153–9.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research 24592018 from the Japan Society for the Promotion of Science. We would like to express sincere appreciation to Drs. M. Kurata, H. Yanagimoto, H. Toyama, Y. Nagakawa, K. Maemura, Y. Mataki, T. Akahori, S. Kinoshita, H. Terashima, A. Horiguchi, Y. Ohtsuka, A. Nanashima, K. Kanemitsu, H. Ohigashi, M. Tani, T. Takahara, H. Shiomi, I. Endo, H. Suzuki, T. Rikiyama, H. Ikoma, M. Yasunaga, K. Nakamura, S. Egawa, Y. Katayose, K. Nakagawa, K. Okada, and S. Ottomo as clinical investigators in the study group of preoperative therapy for pancreatic cancer (PREP).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2018_1506_MOESM1_ESM.pptx
Supplemental Figure 1. Overall survival curve for PDAC planned NAC-GS according to the resectability status. 1A. Survival comparison by intention-to-treat analysis. Median OS of the patients with R PDAC (n=63, solid line) was 39.2 months, which was longer but not significant than that of the patients with BR PDAC (n=38, 21.1 months, broken line)(p=0.35). The 2-year OS of R and BR PDAC were 60.4% and 48.6%, respectively. 1B. Survival comparison by on-protocol analysis. The 2-year OS of R PDAC (n=29, solid line) and BR PDAC (n=15, broken line) were 78.9% and 66.7%, respectively (p=0.70) (PPTX 123 kb)
535_2018_1506_MOESM2_ESM.pptx
Supplemental Figure 2. Overall survival curve for PDAC planned NAC-GS according to cycles receiving postoperative adjuvant treatment. 1A. Survival comparison of on-protocol cohort with or without completion of postoperative adjuvant treatment. The 2-year OS of the patients receiving 6 cycles (n=26, solid line) and less than 6 cycles (n=18, broken line) of gemcitabine adjuvant were 84.4% and 60.6%, respectively (p=0.22). 2B. Survival comparison of on-protocol cohort with 4 and over cycles or less than 4 cycles of postoperative adjuvant treatment. The 2-year OS of the patients receiving 4 and over cycles (n=30, solid line) and less than 4 cycles (n=14, broken line) of gemcitabine adjuvant were 86.5% and 49.0%, respectively (p=0.0067) (PPTX 114 kb)
Rights and permissions
About this article
Cite this article
Motoi, F., Satoi, S., Honda, G. et al. A single-arm, phase II trial of neoadjuvant gemcitabine and S1 in patients with resectable and borderline resectable pancreatic adenocarcinoma: PREP-01 study. J Gastroenterol 54, 194–203 (2019). https://doi.org/10.1007/s00535-018-1506-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-018-1506-7