Abstract
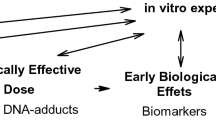
Humans are constantly exposed to a wide range of reactive and toxic chemicals from the different sources in everyday life. Identification of the exposed chemical helps in the detection and understanding the exposure associated adverse health effects. Covalent adducts of proteins and DNA formed after xenobiotics exposure may serve as readily measurable indicators of these exposures. Measuring the exposed chemicals with focus on adducts resulting from the nucleophilic interactions with blood proteins is useful in the development of diagnostic markers. Particularly, the most abundant proteins such as albumin and hemoglobin acts as dominant scavengers for many reactive chemicals in blood and can serve as excellent diagnostic candidates to determine the type of chemical exposure. This review focuses on the potential application of an adductomics approach for the screening of bimolecular adducts of chemical warfare agents (CWAs). Recent incidents of CWAs use in Syria, Malaysia, and the UK illustrate the continuing threat of chemical warfare agents in the modern world. Detection of CWAs and their metabolites in blood or in other body fluids of victims depends on immediate access to victims. Concentrations of intact CWAs in body fluids of surviving victims may decline rapidly within a few days. Certain CWAs, particularly nerve agents and vesicants, form covalent bonds with certain amino acids to form CWA-protein adducts. Proteins that are abundant in the blood, including albumin and hemoglobin, may carry these adducts longer after the original exposure. We searched MEDLINE and ISI Web of Science databases using the key terms “adductomics” “adducts of CWAs,” “CWAs adducts detection in the biological samples,” “protein adducts of CWAs,” alone and in combination with the keywords “detection” “intoxication” “exposure” “adverse effects” and “toxicity.” We also included non-peer-reviewed sources such as text books, relevant newspaper reports, and applicable Internet resources. We screened bibliographies of identified articles for additional relevant studies including non-indexed reports. These searches produced 1931 citations of which only relevant and nonduplicate citations were considered for this review. The analysis of biomedical samples has several purposes including detecting and identifying the type of chemical agent exposed, understanding the biological mechanism, assists in giving adequate treatment, determining the cause of death and providing evidence in a court of justice for forensic investigations. Rapid advances in the mass spectrometry to acquire high-quality data with greater resolution enabled the analysis of protein and DNA adducts of xenobiotics including CWAs and place the rapidly advancing ‘adductomics’ next to the other “-omics” technologies. Adductomics can serve as a powerful bioanalytical tool for the verification of CWAs exposure. This review mostly describes the protein adducts for nerve agents and vesicants, outlines the procedures for measuring adducts, and suggests the evolving (or future) use of adducts in the detection and verification of CWAs.




Similar content being viewed by others
References
Barr JR (ed) (2008) Special issue: analysis of biological samples for chemical warfare agents, J. Anal. Toxicol. Jan-Feb; 32(1):1
Benschop HP, Schans GP, Noort D, Fidder A, de Jong LPA (1997) Verification of exposure to sulfur mustard in two casualties of the Iran–Iraq conflict. J Anal Toxicol 21:249–251
Benschop HP, Noort D, Govert P, Van der Schans, Leo P, De Jong (2000) Diagnosis and dosimetry of exposure to sulfur mustard: development of standard operating procedures; further exploratory research on protein adducts. Prins maurits laboratorium TNO. Dec; 20 Suppl 1:S187–92
Bessonneau V, Pawliszyn J, Rappaport SM (2017) The saliva exposome for monitoring of individuals’ health trajectories. Environ Health Perspect 20:1–10
Black RM (2010) History and perspectives of bioanalytical methods for chemical warfare agent detection. J Chromatogr B. 878:1207–1215
Black RM, Noort D, Mesilaakso M (2005) Chemical weapons convention related analysis, Wiley, Chichester
Black RM, Read RW (2013) Biological markers of exposure to organophosphorus nerve agents. ArchToxicol 87:421–437
Black RM, Clarke RJ, Harrison JM, Read RW (1997) Biological fate of sulfur mustard: identification of valine and histidine adducts in hemoglobin from casualties of sulfur mustard poisoning. Xenobiotica 27:499–512
Carlsson H, Tornqvist M (2017) An adductomic approach to identify electrophiles in vivo. Basic ClinPharmToxicol 121:44–54
Chai PR, Boyer EW, Al-Nahhas H, Erickson TB (2017) Toxic chemical weapons of assassination and warfare: nerve agents VX and Sarin. ToxicolCommun 1(1):21–23
Chai PR, Hayes BD, Erickson TB, Boyer EW (2018) Novichok agents: a historical, current, and toxicological perspective. Toxicol Commun 2(1):45–48
Convention on the Prohibition of the Development (1994) Production, stockpiling and use of chemical weapons and on their destruction, annex on chemicals. Organisation for the Prohibition of Chemical Weapons, The Hague
Davern TJII, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Obaid Shakil A, Lee WM (2006) Acute Liver Failure Study Group. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterol 130:687–694
Europian Union (2002) Commission Decision 2002/657/EC. Off J Eur Commun 221:8–36
Fabisiak JP, Sedloy A, Kagan VE (2002) Quantification of oxidative/nitrosative modification of cys-34 in human serum albumin using a fluorescence-based SDS-PAGE assay.Antioxi. Redox. Sign.: 4; No.5
Farmer PB, Singh R (2008) Use of DNA adducts to identify human health risk from exposure to hazardous environmental pollutants: the increasing role of mass spectrometry in assessing biologically effective doses of genotoxic carcinogens. Mutat Res 659(I1-2):68–76
Fidder A, Hulst AG, Noort D, de Ruiter R, Van der Schans MJ, Benschop HP, Langenberg P (2002) Retrospective detection of exposure to organophosphorus anti-cholinesterases: mass spectrometric analysis of phosphylated human butyryl cholinesterase. Chem Res Toxicol 5:582–590
Funk WE, Pleil JD, Sauter DJ, McDade T, Holl JL (2015) Use of dried blood spots for estimating children’s exposures to heavy metals in epidemiological research. J Environ AnalToxicol. https://doi.org/10.4172/2161-0525.S7-002
Gandor F, Gawlik M, Thiermann H, John H (2015) Evidence of sulfur mustard exposure in human plasma by LC-ESI-MS-MS detection of the albumin-derived alkylated HETE-CP dipeptide and chromatographic investigation of its cis/trans isomerism. J Anal Toxicol 39(4):270–912
Golime R, Bhattacharya BK (2012) Multiple signal transduction pathways alterations during nerve agent toxicity. Toxicol Lett 208:16–22
Golime R, Afley P, Acharya J, Bhattacharya BK (2014) Efficacy of midazolam, atropine and HI-6 on nerve agent induced molecular and neuropathological changes. BMC Neurosci 15:47
Golime R, Palit M, Dubey DK (2018) Neuroprotective efficacy of Galantamine and atropine on nerve agent induced neuroglial and biochemical changes. Neurotox Res May 33(4):738–748
Grigoryan H, Edmands WMB, Lu SS, Yano Y, Regazzoni L, Iavarone AT (2016) An adductomics pipeline for untargeted analysis of modifications to Cys-34 of human serum albumin. Anal Chem 88:10504–10512
Ilse D, Edman P (1963) The formation of 3-phenyl-2-thiohydantoins from phenylthio-carbamyl amino acids. Aust J Chem 16(3):411–416
James LP, Farrar HC, Sullivan JE, Givens TG, Kearns GL, Wasserman GS, Walson PD, Hinson JA, Pumford NR (2001) Measurement of acetaminophen-protein adducts in children and adolescents with acetaminophen overdoses. J Clin Pharmacol 41(8):846–851
John H, David J in: Jenner J, Thiermann H, Worek F (eds) (2016) The impact of new technologies on the elucidation of CWA toxicology from the book Chemical Warfare Agents Toxicology. Vol.2.17 May 2016. Issues in Toxicol no. 27. Royal. Soc.Chem
John H, Siegert M, Willoh S, Hormann P, Thiermann H (2016a) Novel procedures for analysis of dried plasma using microsampling devices to detect sulfur mustard-albumin adducts for verification of poisoning. Anal Chem 88(17):8787–8794
John H, Siegert M, Gandor F, Gawlik M, Kranawetvogl A, Karaghiosoff K (2016b) Optimized verification method for detection of albumin-sulfur mustard adduct at Cys-34 using a hybrid quadrupole time-of-flight tandem-mass spectrometer after direct plasma proteolysis. Toxicol Lett 244:13,103–111
John H, van der Schans MJ, Koller M, Spruit HET, Worek F, Thiermann H, Noort D (2018) Fatal sarin poisoning in Syria 2013: 2018. Forensic verification within an international laboratory network. Foren.Toxicol;36:61–71
Jowsey PA, Faith M, Williams, Blain PG (2009) DNA damage, signalling and repair after exposure of cells to the sulphur mustard analogue 2-chloroethyl ethyl sulphide. Toxicol 257(3):105–112
Kanaly RA, Hanaoka T, Sugimura H, Toda H, Matsui S, Matsuda T (2006) Development of the adductome approach to detect DNA damage in humans. Antioxi Redox Sign 8:993–1001
Koc H, Swenberg JA (2002) Applications of mass spectrometry for quantitation of DNA adducts. J Chromatogr B 778:323–343
Kranawetvogl A, Worek F, Thiermann H, and John H (2016) Modification of human serum albumin by the nerve agent VX: microbore liquid chromatography/electrospray ionization high-resolution time-offlight tandem-mass spectrometry method for detection of phosphonylated tyrosine and novel cysteine containing disulfide adducts. Rapid Commun Mass Spectrom 30(19):2191–2200
Kranawetvogl A, Kuppers J, Gütschow M, Worek F, Thiermann H, Elsinghorst PW, John H (2017) Identification of novel disulfide adducts between the thiol containing leaving group of the nerve agent VX and cysteine containing tripeptides derived from human serum albumin. Drug Test Anal 9(8):1192–1203
Kranawetvogl A, Kuppers J, Siegert M, Gutschow M, Worek F, Thiermann H, Elsinghorst PW, John H (2018) Bioanalytical verification of V-type nerve agent exposure: simultaneous detection of phosphonylated tyrosines and cysteine-containing disulfide-adducts derived from human albumin. AnalBioChem 410:1463–1474
Mattes WB, Hartley JA, Kohn KW (1986) DNA sequence selectivity of guanine-N7 alkylation by nitrogen mustards. Nucleic Acids Res 14:2971–2987
Mesilaakso M (2004) Chemical weapons convention chemical analysis—sample collection, preparation and analytical methods. Wiley, Chichester
Miyaki K, Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Yoshimura K, Etoh N, Matsumoto Y, Kikuchi Y, Kumagai N, Omae K (2005) Effects of sarin on the nervous system of subway workers seven years after the Tokyo subway sarin attack. J Occup Health 47:299–304
Nie Z, Liu Q, Xie J (2011) Improvements in monitoring the N-terminal valine adduct in human globin after exposure to sulfur mustard and synthesis of reference chemicals. Talanta 85:21154–21159
Niu Tian-qi, Matijasevic Z, Austin-Ritchie P, Stering A, Ludlum DB (1996) A 32P-postlabeling method for the detection of adducts in the DNA of human fibroblasts exposed to sulfur mustard. Chem Biol Interact 100(1):77–84
Noort D, Hulst AG, Trap HC, de Jong LP, Benschop HP (1997) Synthesis and mass spectrometric identification of the major amino acid adducts formed between sulphur mustard and hemoglobin in human blood. Arch Toxicol 71:171–178
Noort D, Benschop HP, Black RM (2002) Biomonitoring of exposure to chemical warfare agents: a review. Toxicol Appl Pharmacol 184:116–126
Noort D, Fidder A, Degenhardt-Langelaan CE, Hulst AGJ (2008) Retrospective detection of sulfur mustard exposure by mass spectrometric analysis of adducts to albumin and hemoglobin: an in vivo study. J Anal Toxicol 32:25–30
Organisation for the Prohibition of Chemical Weapons (OPCW). Guidelines for the Biomedical Sample Analysis Exercise. 2015, Available from the OPCW Laboratory
Organization for the Prohibition of Chemical Weapons (OPCW). Technical Secretariat for designating laboratories for the analysis of authentic biomedical samples. 2018, CS-2018-1264(E) distributed 14/08/2018.Available from the OPCW Laboratory
Osborne MR, Wilman DE, Lawley PD (1995) Alkylation of DNA by the nitrogen mustard bis (2-chloroethyl) methylamine. Chem Res Toxicol 8:316–320
Ozbal CC, Skipper PL, Yu MC, London SJ, Dasari RR (2000) Quantification of (7S,8R)-dihydroxy- (9R,10S)-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene adducts in human 87 serum albumin by laser-induced fluorescence: implications for the in vivo metabolism of benzo[a]pyrene. Cancer Epidemiol Biomark Prev 9:733–739
Perez JW, Pantazides BG, Watson CM, Thomas JD, Blake TA, Johnson RC (2015) Enhanced stability of blood matrices using a dried sample spot assay to measure human butyrylcholinesterase activity and nerve. Agent Adducts Anal Chem 87:5723–5729
Polhuijs M, Langenberg JP, Benschop HP (1997) New method for retrospective detection of exposure to organophosphorus anticholinesterases: application to alleged sarin victims of Japanese ter-rorists. Toxicol Appl Pharm 146:156–161
Rao PV, Lakshmana R, Vijayaraghavan, Bhaskar ASB (1999) Sulphur mustard induced DNA damage in mice after dermal and inhalation exposure. Toxicol 139:1–2
Rappaport SM, Li H, Grigoryan H, Funk WE, Williams ER (2012) Adductomics: characterizing exposures to reactive electrophiles. Toxicol Lett 213:83–90
Skipper PL, Tannenbaum SR (1990) Protein adducts in the molecular dosimetry of chemical carcinogens. Carcinogenesis 11:507–518
Steinritz D, Striepling E, Rudolf KD, Schroder-Kraft C, Puschel K, Hullard-Pulstinger A et al (2016) Medical documentation, bioanalytical evidence of an accidental human exposure to sulfur mustard and general therapy recommendations. Toxicol Lett 244:112–120
Tornqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P (2002) Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J Chromatogr B 778:279–308
Tretyakova N, Goggin M, Sangaraju D, Janis G (2012) Quantitation of DNA adducts by stable isotope dilution mass spectrometry. Chem Res Toxicol 25:no.10: 2007–2035
U.S. Department of Health and Human Services, Food and Drug Administration, Guidance for Industry, Bioanalytical Method Validation. http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf. Accessed 23 May 2015
United Nations Mission to Investigate (2013) Allegations of the use of chemical weapons in the Syrian Arab Republic, Final report, A68/663, S/2013/735
Vale JA, Marrs TC, Maynard RL.Novichok (2018) A murderous nerve agent attack in the UK. Clin Toxicol 56:11:1093–1097
Van der Schans GP, Mars-Groenendijk R, De Jong LPA, Benschop HP, Noort D (2004) Standard operating procedure for immunuslotblot assay for analysis of DNA/sulfur mustard adducts in human blood and skin. J Anal Toxicol 28(5):316–319
Van der Schans MJ, Fidder A, van Oeveren D, Hulst AG, Noort D (2008) Verification of exposure to cholinesterase inhibitors: generic detection of OPCW Schedule 1 nerve agent adducts to human butyrylcholinesterase. J Anal Toxicol 32:125–130
World Anti-Doping Agency, Technical Document WADA TD2010IDCR (2010) https://www.wada-ama.org/en/resources/science-medicine/td2010-idcr. Accessed 23 May 2015
Zubel T, Hochgesand S, John H, Steinritz D, Schmidt A, Bürkle A, Mangerich A (2019) A mass spectrometric platform for the quantitation of sulfur mustard-induced nucleic acid adducts as mechanistically relevant biomarkers of exposure. Arch Toxicol 93(1):61–79
Acknowledgements
The authors thank Director, and DRDE, Gwalior for providing necessary facilities and encouragement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no financial or personal conflicts of interest in this publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Golime, R., Chandra, B., Palit, M. et al. Adductomics: a promising tool for the verification of chemical warfare agents’ exposures in biological samples. Arch Toxicol 93, 1473–1484 (2019). https://doi.org/10.1007/s00204-019-02435-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-019-02435-4