Abstract
ABP 501 [United States: AMJEVITA™ (adalimumab-atto); European Union: AMGEVITA® (adalimumab)] is the first approved biosimilar to adalimumab [reference product (RP)], a monoclonal antibody (mAb) targeting tumor necrosis factor-alfa (TNF-α). ABP 501 has received approval for use in indications that adalimumab RP is approved for, except those protected by regulatory exclusivity. A systematic step-wise totality of evidence (TOE) approach formed the basis of approval of ABP 501; this involved methodical accumulation of scientifically robust comparative data supporting similarity in analytical, preclinical, and clinical [pharmacokinetics (PK)], efficacy, safety and immunogenicity) evaluations. As a foundational first step, comprehensive analytical assessments demonstrated that ABP 501 is structurally and functionally similar to adalimumab RP in critical quality attributes. Preclinical assessments confirmed similar activity in assessing mechanisms of action and toxicology. Clinical evaluation included a phase 1 PK equivalence study in healthy subjects and two comparative phase 3 studies that evaluated ABP 501 and adalimumab RP in two sensitive patient populations, plaque psoriasis (PsO) and rheumatoid arthritis (RA). The PK profiles of ABP 501 and adalimumab RP were similar in healthy subjects as well as patients with PsO and RA. The pivotal phase 3 study in patients with PsO demonstrated that ABP 501 was clinically similar to adalimumab RP in terms of efficacy, safety and immunogenicity in both the primary and transition phases. The pivotal phase 3 study in patients with RA also established clinical similarity between ABP 501 and adalimumab RP; an open-label extension of this study demonstrated sustained efficacy over an additional 72 weeks, with no new safety or immunogenicity concerns with ABP 501 treatment. Overall, the TOE supported the conclusion that ABP 501 is highly similar to adalimumab RP and provided scientific justification for extrapolation to all the approved indications of adalimumab RP not protected by exclusivities.
Funding: Amgen Inc.
Similar content being viewed by others
Introduction
ABP 501 [United States (US): AMJEVITA™ (adalimumab-atto); European Union (EU): AMGEVITA® (adalimumab); Amgen Inc., Thousand Oaks, CA, USA] is the first approved biosimilar to adalimumab (HUMIRA®; AbbVie Inc., North Chicago, IL, USA), a human immunoglobulin G1 (IgG1) monoclonal antibody (mAb) that specifically binds to both soluble (sTNFα) and membrane-bound tumor necrosis factor-alfa (mbTNFα), a pleotropic proinflammatory cytokine that plays a central role in the pathogenesis of multiple inflammatory diseases. Depending on the target cell engaged, binding of soluble TNFα with its cognate cell surface receptors, TNF receptor 1 and TNF receptor 2, leads to induction of either gene expression via the nuclear factor kappa B-mediated pathway and production of inflammatory cytokines or cell death [1]. The primary mechanism of action of adalimumab is binding and neutralization of soluble TNFα, thereby blocking subsequent TNFα signaling and the promotion of inflammation. Moreover, the binding of adalimumab to membrane-bound TNFα also triggers induction of effector functions including antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) as well as induction of reverse signaling, which may be relevant for the observed efficacy of adalimumab in patients with inflammatory bowel disease (IBD) [2].
ABP 501 is approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) for all the requested indications of adalimumab (HUMIRA®; AbbVie, Chicago, IL, USA; AbbVie, Ludwigshafen, Germany); a few indications were not requested due to regulatory exclusivity [3, 4]. In the US, the indications include moderately to severely active rheumatoid arthritis (RA), moderately to severely active juvenile idiopathic arthritis (in patients aged 4 years and older), psoriatic arthritis (PsA), ankylosing spondylitis (AS), adult moderately to severely active Crohn’s disease (CD), moderately to severely active ulcerative colitis (UC), and moderate to severe chronic plaque psoriasis (PsO) [3]. In the EU, ABP 501 is additionally approved for two other indications, i.e., moderate to severe hidradenitis suppurativa and adult non-infectious intermediate, posterior and panuveitis [4].
A biosimilar is a biological therapeutic that is highly similar to an already approved originator reference product (RP). Different regulatory authorities consistently define biosimilars as being highly analytically similar to the originator RP, with no clinically meaningful differences in purity, potency, pharmacokinetics (PK), pharmacodynamics (PD), clinical efficacy, safety and immunogenicity, notwithstanding the presence of minor differences in clinically inactive components [5,6,7].
Biosimilars are a distinct category of products different from small molecule generic drugs [5, 6, 8]. Whereas generics are chemically synthesized and identical to their RP, biologics such as mAbs and recombinant proteins are structurally and functionally complex entities that require development of unique cell lines and manufacturing processes in living systems. Thus, biosimilars are “similar” to their RP; it is impossible to produce biosimilars that are identical to the originator RP. Because the proprietary manufacturing processes of originator RP are not known, it is important to characterize multiple lots of RP and to understand the critical quality attributes (CQAs) to match. This characterization aids in designing a biosimilar that has similar identified CQAs, through optimization of the unique cell line and manufacturing processes used by the biosimilar manufacturer. During the normal manufacturing process of biologics, post-translational modifications occur on the protein produced in a living cell system; such modifications can confer minor structural, and in some cases, functional differences in the biosimilar molecule. These modifications, although minor, should be evaluated for potential impact of functional similarity [5, 6]. For the establishment of biosimilarity, the biosimilar must demonstrate that any structural differences do not confer clinically meaningful differences and that there is no gain of new functionality. Moreover, biologics have an intrinsic potential for immunogenicity, which should be specifically evaluated during clinical development, as there are currently no in vitro assessments capable of predicting clinical immunogenicity.
Due to the complexity of biologics production and characterization, the regulatory pathway for biosimilars is different from that for generics; US and EU biosimilar guidance recommend that biosimilars demonstrate similarity based on a step-wise totality of evidence (TOE) approach, which begins with the demonstration of analytical (structural and functional) similarity followed by preclinical pharmacology and clinical PK and PD (if applicable) evaluations; and finally, clinical efficacy, immunogenicity, and safety as compared to the RP [5, 6, 8]. Building this TOE occurs in several stages (Fig. 1). The first step involves a comprehensive analytical and functional characterization that serves as the foundation for the establishment of biosimilarity. A meaningful assessment of analytical similarity requires extensive and robust studies using multiple state-of-the-art analytical techniques and a full evaluation of all known and plausible mechanisms of action of the RP. The next step may involve preclinical assessments, particularly if there are uncertainties regarding observed structural and/or functional differences that a preclinical study could address. Such preclinical assessments could include animal studies to compare the toxicology, toxicokinetics, or PD characteristics of the biosimilar as compared to the RP with the goal of reducing any residual uncertainty remaining from the analytical similarity assessment. This is then followed by assessment of phase 1 comparative human PK (and PD where applicable) studies; the final step is confirmatory clinical evaluation of the proposed biosimilar in a sensitive patient population to demonstrate similar efficacy, immunogenicity and safety as compared to the RP. A robust analytical characterization with minimal differences in structural and functional assessments provides justification for reduced regulatory requirements for clinical evaluations, which allows the program to proceed directly to phase 3 comparative clinical evaluations after demonstration of PK/PD biosimilarity.
This review presents the step-wise process that was used to generate the robust TOE leading to the approval of ABP 501 as the first biosimilar to adalimumab. The adalimumab RP used for the analytical and clinical PK assessments presented in the current review was acquired from the US and EU. This was necessary because RP is defined as that approved in the local jurisdiction (i.e., US and EU); thus, ABP 501 must be separately shown to be similar to the adalimumab RP approved in the US and EU. However, recognizing the complexity and cost associated with fulfilling such a requirement, both regulatory agencies allow the use of foreign-sourced comparators in comparative clinical studies, contingent upon establishing a scientific rationale or “scientific bridge” that demonstrates that the foreign-sourced RP is similar to the locally-sourced RP in analytical and clinical PK/PD assessments [5, 6]. This then provides sufficient justification for the use of a single comparator in the confirmatory clinical study(ies).
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Analytical Characterization
Similarity assessments start with risk-ranking the CQA based on scientific understanding of how each product quality attribute impacts safety, efficacy, immunogenicity, and/or PK and PD. The testing strategy and evaluation approaches are designed to incorporate state-of-the-art analytical methods, and assessment criteria are established considering the criticality of the attribute being measured. Statistical considerations are used to establish objective assessment criteria to aid in the interpretation of biosimilarity. The result is a scientifically justified analytical similarity assessment that demonstrates that a proposed biosimilar is structurally and functionally similar to a RP.
The ABP 501 similarity assessment testing plan was designed to comprehensively assess analytical and functional similarity and ensure the detection and understanding of any differences between ABP 501 and the adalimumab RP [9]. Physicochemical similarity of adalimumab RP and ABP 501 was demonstrated through extensive analytical characterization incorporating orthogonal analytical techniques to assess product quality attributes in multiple categories, including general properties, primary structure including amino acid sequence and post-translational modifications, higher-order structure, purity, product- and process-related impurities, particles and aggregates, and thermal-forced degradation profiles [9]. To ensure an objective conclusion of structural similarity between ABP 501 and the adalimumab RP, comprehensive statistical approaches were used to confirm similarity [10]. Functional similarity was evaluated for all established and plausible mechanisms of action of ABP 501 and adalimumab RP using primary (potency) and orthogonal measures to support that the minor analytical differences observed did not impact the activity of ABP 501 [11].
Structural Similarity
The primary structure of ABP 501 was shown to be similar to the adalimumab RP based on comparative assessments including peptide mapping which confirmed identical amino acid sequences (Fig. 2a). Post-translational modifications including C‐ and N‐terminus modification, deamidation, and oxidation were detected among the products at similar levels [9]. Moreover, glycan mapping established that ABP 501 and the adalimumab RP had similar glycosylation profiles in terms of major glycans important to functional activities and PK characteristics, including afucosylated and high mannose glycans (Fig. 2b).
Structural similarity: comparison of primary structure of ABP 501 with adalimumab (US and EU) [9]: a reduced peptide map. b glycan map
The higher-order structure was also found to be highly similar between ABP 501 and adalimumab RP [9]. In addition, assessments of levels and characteristics of different sizes of particles and aggregates, which identifies any subvisible and submicron particles and impurities that could impact immunogenicity and safety concerns, were determined to be low and similar for APB 501 and adalimumab RP. Product- and process-related impurities that result from the different manufacturing processes were also found to be similar. Analyses of relevant host-cell impurities, including host-cell protein and host-cell DNA, were found to be well controlled for ABP 501. Overall, general properties of ABP 501 and adalimumab RP, including protein concentration and volume, all met the pre-specified criteria for analytical similarity. Due to the different formulation of ABP 501 as compared with the adalimumab RP (of note, the regulatory guidance allows for a different formulation for proposed biosimilars [5, 6]), thermal degradation behavior of ABP 501 was found to be non-inferior compared to that of adalimumab RP, as evidenced by decreased formation of high molecular weight species in ABP 501 compared to adalimumab under forced degradation conditions. Taken together, these results from the similarity assessment demonstrated that ABP 501 was highly analytically similar to adalimumab RP.
Functional Similarity
As a critical component of the TOE for biosimilar development, functional similarity assessments of ABP 501 versus adalimumab RP were conducted. As the primary mechanism of action of adalimumab and ABP 501 is the binding and neutralization of sTNFα, this activity was measured in multiple assays, including those resulting in caspase activation, cytokine and chemokine production, and in cell death [11].
The results demonstrated similarity between ABP 501 and adalimumab RP in binding to both sTNFα and mbTNFα; these results were further confirmed by comparing the binding kinetics of ABP 501 and adalimumab RP using surface plasmon resonance. Both ABP 501 and adalimumab RP showed similarity in neutralization of sTNFα activity, reflecting the inhibition of sTNFα-induced proinflammatory signaling. The potency of ABP 501 was demonstrated to be statistically equivalent to adalimumab RP, as assessed by inhibition of TNFα-induced apoptosis (Fig. 3a). ABP 501, adalimumab (US), and adalimumab (EU) were also similar in terms of inhibition of TNFα-induced IL-8 secretion, with EC50 values ranging from 192 to 294 pM, 131 to 253 pM, and 168 to 225 pM, respectively (Fig. 3b). Moreover, ABP 501 and adalimumab RP were unable to neutralize lymphotoxin alpha bioactivities, the most closely related cytokine to TNFα, indicating specificity for TNFα with no gain of function for ABP 501.
Functional similarity: comparison of ABP 501 with adalimumab (US and EU): a potency: inhibition of TNFα-induced apoptosis [11]; b potency: inhibition of TNFα-induced IL-8 secretion [11]; c effector function activity: induction of ADCC [11]; d effector function activity: induction of CDC [11]; e reverse signaling [12]
Given that adalimumab is an IgG1 and mediates effector functions (ADCC and CDC) that may play a role in efficacy in the IBD indications, an evaluation of Fc receptor binding profiles and effector function was conducted, which included binding to Fc-gamma receptors FcγRIa, FcγRIIa (131H), FcγRIIIa (158V and 158F), and neonatal Fc receptor (FcRn) [11]. Importantly, to confirm similarity in activation of Fc-mediated effector function, ABP 501 and adalimumab RP were demonstrated to be similar in terms of induction of ADCC and CDC (Fig. 3c, d) [11].
Additionally, binding to mbTNFα can also result in other outcomes including inhibition of proliferation in a mixed lymphocyte reaction (MLR) through induction of regulatory macrophages, in addition to reverse signaling and apoptosis of mbTNFα expressing immune cells; thus, these activities were also assessed [12]. Both ABP 501 and adalimumab RP were shown to similarly inhibit proliferation in an MLR assay and showed similar mean relative activity in reverse signaling (Fig. 3e).
Overall, the comprehensive assessment of functional activity, including all established and plausible mechanisms of action of adalimumab, demonstrated high similarity between ABP 501 and adalimumab RP. The similar functional activity of ABP 501 supports the TOE for biosimilarity and the scientific justification for extrapolation to all non-studied indications approved for adalimumab.
Non-Clinical Studies in Vivo
A repeat dose toxicology study was conducted with ABP 501 and adalimumab (US) RP in the cynomolgus monkey model. In this study of 1-month duration, weekly doses of ABP 501 and the adalimumab RP showed similar expected lymphoid changes, which included decreased size and number of germinal centers in axillary lymph nodes, mesenteric lymph nodes, and tonsil. Repeat-dose toxicokinetics were also conducted as part of the toxicology study (unpublished data, Amgen Inc.). Mean toxicokinetic parameters and serum concentration-time profiles at day 1 and again following the last dose were similar between ABP 501 and adalimumab RP.
Clinical Pharmacology
To further confirm similarity, a phase 1 randomized, single-blind, single-dose, three-arm, parallel-group study was conducted to demonstrate the PK equivalency of ABP 501 with adalimumab RP after administration of a single 40-mg subcutaneous (SC) dose in healthy subjects [13]. Healthy volunteers provided the most homogenous population to detect any differences between ABP 501 and the adalimumab RP. The adalimumab RP in this study was acquired from both EU (adalimumab EU) and the US (adalimumab US) to complete the scientific bridge described above. Primary endpoints were area under the serum concentration–time curve from time 0 extrapolated to infinity (AUCinf) and the maximum observed serum concentration (Cmax); secondary objectives were assessments of safety, tolerability, and immunogenicity.
A total of 203 healthy male and female adults were dosed and included in the safety and PK analyses. The geometric mean ratios (GMRs) for PK parameters were similar between ABP 501 and adalimumab (US) as well as between ABP 501 and adalimumab (EU). The 90% confidence intervals (CIs) for the GMRs of AUCinf and Cmax were within the protocol-specified standard PK equivalence criteria of 0.80–1.25, indicating PK similarity between ABP 501 and adalimumab (US) as well as ABP 501 and adalimumab (EU) (Fig. 4).
ABP 501 phase 1 study: PK results [23]
Treatment-emergent adverse events (TEAEs), regardless of causality, were mild to moderate and were balanced between the treatment groups. The most common TEAEs were headache, oropharyngeal pain, sinus congestion, nasopharyngitis, and nausea. In terms of immunogenicity, the incidence of anti-drug antibodies (ADAs) was similar among the study groups. PK parameters were affected by the ADA status, with a decrease in the study drug half-life reported in ADA-positive subjects compared with ADA-negative subjects; however, the impact was similar for both ABP 501 and the adalimumab RP [13]. In addition, population PK modeling confirmed the PK similarity of ABP 501 and adalimumab RP for both ADA-negative and ADA-positive subjects [14]. Overall, these results demonstrated PK similarity between ABP 501 and adalimumab RP.
Clinical Evaluation
The final step that completes the TOE for biosimilarity demonstration is clinical confirmation of similar efficacy, safety, and immunogenicity of ABP 501 with the adalimumab RP.
Two randomized phase 3 clinical studies were conducted for ABP 501, one in patients with moderate to severe PsO (NCT01970488) and the other in patients with moderate to severe RA also receiving methotrexate (NCT01970475). In addition, the RA study was followed by an open-label extension (OLE) study that evaluated the safety and efficacy of ABP 501, such that the total evaluation of the safety of ABP 501 reached 2 years of treatment (NCT02114931). A single comparator was used in these clinical studies: EU-authorized RP in the PsO study and FDA-licensed RP in the RA study. This is because, as discussed earlier, a scientific bridge was considered to have been established during the analytical and phase 1 PK evaluations, thereby justifying the use of a single RP in the ABP 501 clinical program.
Demonstration of Clinical Equivalence in Moderate to Severe Plaque Psoriasis
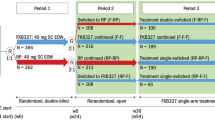
This was a randomized, double-blind, multicenter, active-controlled, phase 3 trial, that included a 4-week screening period, followed by 1:1 randomization to receive ABP 501 or adalimumab RP treatment for 16 weeks [15, 16]. Patients were administered ABP 501 and adalimumab RP at an initial loading dose of 80 mg subcutaneously (SC) on week 1/day 1, followed by 40 mg SC every 2 weeks (starting at week 2) for 16 weeks. Patients with 50% improvement from baseline in psoriasis area and severity index score (PASI 50) at week 16 remained on the study and were rerandomized 1:1 to continue on study until 52 weeks. Patients who received ABP 501 in the first randomization continued ABP 501 treatment, and those who received adalimumab RP initially were rerandomized to either continue adalimumab RP or switch to ABP 501. All patients received assigned treatment every 2 weeks until week 48, followed by a 4-week safety follow-up period. The final efficacy assessments were performed at week 50; the end-of-study visit was at week 52.
Eligible patients had stable moderate to severe PsO for ≥ 6 months, had inadequately responded to or were unable to tolerate/receive ≥ 1 conventional systemic therapy, had disease involvement of ≥ 10% of body surface area (BSA), a PASI score ≥ 12 and a static Physician’s Global Assessment (sPGA) of at least moderate severity (sPGA range: 0–5) [15, 16].
The primary efficacy endpoint was the percent improvement in PASI score from baseline to week 16. Secondary endpoints included PASI percent improvement from baseline at week 32 and week 50; PASI 50, ≥ 75% improvement in PASI score from baseline (PASI 75), ≥ 90% improvement in PASI score from baseline (PASI 90), and 100% improvement in PASI score from baseline (PASI 100) responses; sPGA response of clear (0) or almost clear (1); and mean change in affected BSA from baseline at weeks 16, 32, and 50 [15, 16]. Safety assessments included monitoring for TEAEs and serious adverse events (SAEs), laboratory data, vital signs, and immunogenicity.
A total of 350 patients were randomized on study (ABP 501, n = 175; adalimumab, n = 175) and a total of 326 patients completed the trial to week 16. At week 16, 308 patients were rerandomized to continue through to week 52 (ABP 501/ABP 501, n = 152; adalimumab RP/adalimumab RP, n = 79; adalimumab RP/ABP 501, n = 77).
Primary Efficacy and Safety Analysis Results: Initial 16-Week Period
At week 16, the percent improvement in PASI score from baseline was similar between the two groups, with a least-square mean difference of − 2.18 (95% CI, − 7.39 to 3.02) (Fig. 5a) [15]. Since the 95% CIs of the primary endpoint were within the prespecified margin (− 15 to 15), clinical equivalence between ABP 501 and adalimumab RP was demonstrated. Key secondary efficacy endpoints, including the proportion of patients achieving PASI 50, PASI 75, PASI 90, PASI 100, sPGA clear/almost clear, and BSA affected at week 16, were also similar between the two treatment groups.
During the initial 16-week treatment period, the incidences of TEAE (67.2% vs. 63.6%) and discontinuations due to TEAEs (4% vs. 2.9%) were similar between the ABP 501 and adalimumab RP groups (Table 1) [15]. AEs occurring in ≥ 5% of patients in any treatment group were nasopharyngitis, headache, and upper respiratory tract infection. Other TEAEs of interest included infections, serious infections, hypersensitivity, and injection-site reactions.
A sub-analysis of patients with a medical history of psoriatic arthritis also showed that the efficacy, safety, immunogenicity, and PK for ABP 501 and adalimumab RP were similar and consistent with the primary study results [17].
Single Transition Results: 16–52 Weeks
Following the single planned switch from adalimumab RP to ABP 501 at week 16, the rerandomized patients achieved similar mean PASI percent improvement from baseline to week 16 in the ABP 501/ABP 501, adalimumab RP/adalimumab RP, and the adalimumab RP/ABP 501 groups [16]. In the rerandomized population, percent improvement in PASI, PASI 50/75/90/100 responses were similar across the three groups from week 16 to the end of the study (week 52), with no significant difference between groups (Fig. 5b). Also, sPGA positive responses achieved in the transition phase were similar at all timepoints, across all groups, and to the pre-switch results. Similarly, among the rerandomized population, changes from baseline in percent BSA were similar across the three groups at weeks 16, 32, and 50.
After week 16 and through week 52 (following rerandomization), similar percentages of patients experienced ≥ 1 TEAE between the three groups (Table 1) [16]. Imbalances in ≥ 5% TEAEs were noted for nasopharyngitis, headache, diarrhea and back pain. The most common AEs of interest were nasopharyngitis and upper respiratory tract infection. Overall, events of hypersensitivity were low and included contact dermatitis (ABP 501/ABP 501, n = 4), all of which were noted to be unrelated to treatment, rash (ABP 501/ABP 501, n = 3) and urticaria (adalimumab RP/ABP 501, n = 2). These did not represent any clinically meaningful differences between treatment groups. Changes in liver enzymes occurred in 13 patients—the group that continued on ABP 501 after rerandomization (ABP 501/ABP501 group) had a greater proportion of patients with increased liver enzymes including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyltransferase (GGT) in contrast to the other two groups (adalimumab RP/ABP 501 and adalimumab RP/adalimumab RP). In the latter two groups, changes in liver enzymes were grade 1 or 2 in severity and were resolved by the end of study in all but one case of elevated GGT. Injection site reactions were noted in five patients and appeared to be comparable between the ABP 501/ABP 501 and adalimumab RP/adalimumab RP groups; the majority of these events were ascribed to injection-site pain. Over the course of the study, no deaths and no cases of demyelinating disease, heart failure, or lupus-like syndromes were reported.
Demonstration of Clinical Equivalence in Moderate to Severe Rheumatoid Arthritis
A randomized, double-blind, active comparator-controlled equivalence study was conducted to compare the clinical efficacy, safety, and immunogenicity of ABP 501 with adalimumab RP and to establish clinical similarity in adalimumab-naive adult patients with moderate to severe RA who had an inadequate response to methotrexate (MTX) [18]. This study included patients with a diagnosis of moderate to severe RA [per 2010 American College of Rheumatology/European League Against Rheumatism criteria (ACR/EULAR) criteria] for ≥ 3 months, active RA (≥ 6 swollen joints and ≥ 6 tender joints) at screening and baseline, an erythrocyte sedimentation rate ≥ 28 mm/hour serum C-reactive protein (CRP) > 1.0 mg/dL with positivity for rheumatoid factor or anticyclic citrullinated peptide antibodies at screening. Additionally, patients must have received MTX (dose of 7.5–25 mg/week) for ≥ 12 consecutive weeks before receiving the investigational drug, which they continued for the study duration. Enrolled patients were randomized 1:1 to receive either ABP 501 or adalimumab RP 40 mg SC on day 1 and then every 2 weeks until week 22. Assessments for the primary endpoint occurred at week 24. Safety and immunogenicity assessments were made at week 26, while ADA levels were measured at baseline and at weeks 4, 12, and 26. The primary efficacy endpoint was the risk ratio (RR) of achieving a 20% improvement from baseline in the American College of Rheumatology (ACR) core set of measurements (ACR20) at week 24. Secondary efficacy endpoints included assessments of disease activity score 28-joint count-CRP (DAS28-CRP), the RR for achieving 20% (ACR20), 50% (ACR50), and 70% (ACR70) improvement in ACR core set of measurements at various time points throughout the study. Other endpoints assessed in this study included the risk differences (RD) for ACR20, ACR50, and ACR70. Key measures for safety assessment included TEAEs, SAEs and incidence of ADAs.
A total of 526 patients were randomized and treated with study drug (ABP 501, n = 264; adalimumab RP, n = 262) [18]. Baseline demographics and clinical characteristics were similar between groups; mean baseline DAS28-CRP scores were 5.66 for the ABP 501 group and 5.68 for the adalimumab RP group. The majority of patients were female (81.0%) and white (95.1%), and mean age was 55.9 years (range: 21–80 years) with a mean of 9.39 years since RA diagnosis. Prior use of biologics for RA and baseline use of RA medications was balanced across groups; a majority of patients in both groups (ABP 501, 73.1%; adalimumab RP, 71.8%) were treatment-naive for prior use of biologics for RA.
Clinical Efficacy
At week 24, a similar proportion of patients in the ABP 501 (74.6%) and adalimumab RP (72.4%) groups met the ACR20 response criteria, with an RR (two-sided 90% CI) of 1.039 (0.954, 1.133) for ABP 501 versus adalimumab RP (Fig. 6) [18]. The 90% CI (0.954, 1.133) fell within the predefined equivalence margin (0.738, 1.355), demonstrating clinical similarity between ABP 501 and adalimumab RP. In addition, the RD for ACR20 at week 24 between the ABP 501 and adalimumab RP groups was 2.604 (two-sided 90% CI, − 3.728, 8.936). The proportion of ACR20 responders was comparable between the two treatment groups at weeks 2 and 8.
Phase 3 trial data in moderate to severe RA: percentage of patients achieving ACR20, ACR50, and ACR75 [18]
A similar proportion of patients achieved ACR50 and ACR70 response criteria at week 24 with ABP 501 versus adalimumab RP (Fig. 6). The percentage of ACR50 and ACR70 responders was similar between treatment groups throughout the study. Additionally, the mean change from baseline in DAS28-CRP was − 2.32 for both ABP 501 and adalimumab RP groups at week 24. A similar decrease in mean change from baseline in DAS28-CRP was noted in both groups throughout the study, indicating similarly reduced disease activity. For both groups, a greater percentage of patients achieved DAS28-CRP remissions over time from weeks 2 to 18 (range, ABP 501: 6.3–31.1%; adalimumab RP: 2.8–27.1%). At week 24, 30.5% in the ABP 501 group and 35.5% of patients in the adalimumab RP group reached the criteria of DAS28-CRP remission, further substantiating clinical efficacy equivalence between ABP 501 and the adalimumab RP.
Safety
Overall, similar proportions of patients reported TEAEs in the ABP 501 and adalimumab RP groups (50.0% vs. 54.6%) (Table 2) [18]. TEAEs reported by ≥ 3% of patients were nasopharyngitis (6.4% vs. 7.3%), headache (4.5% vs. 4.2%), arthralgia (3.0% vs. 3.4%), cough (2.7% vs. 3.1%), and upper respiratory tract infection (1.5% vs. 3.8%). A total of 27 SAEs in 23 patients were reported throughout the study, with similar incidence in the ABP 501 (n = 10; 3.8%) and adalimumab RP (n = 13; 5.0%) groups. Sepsis (n = 2; ABP 501) was the only SAE reported for > 1 patient, which resolved by the end of the study. There were no deaths in this study. Adverse events of interest that occurred during the study were mostly of grade 1 or 2 severity and included infections, malignancies, hypersensitivity, and liver enzyme elevations (Table 2). Two malignancies (basal cell carcinoma and squamous cell carcinoma) were reported in one subject in the ABP 501 group, and another (squamous cell carcinoma of the skin) was reported in one subject in the RP group. Events of hypersensitivity occurring in > 2 patients included rash, erythematous rash, and allergic dermatitis. Overall, 2.3% of patients in the ABP 501 and 5.0% of patients in the adalimumab groups experienced injection-site reactions. Although elevations in liver enzymes were reported, none led to premature study discontinuation and none were associated with increases in bilirubin.
Open-Label Extension Study in Moderate to Severe RA
An open-label, single-arm extension of the phase 3 study in RA (NCT02114931, Parent Study) was designed to further evaluate the safety, efficacy, and immunogenicity for an additional 72 weeks in patients with moderate to severe RA [19]. All subjects who had completed the week 26 visit received ABP 501 40 mg every other week starting on day 1 of the OLE study. Primary outcomes were the incidence of TEAEs, severe adverse events (SAEs), clinically significant changes in laboratory analytes, and ADA incidence. Secondary outcomes included the percentage of participants with an ACR20 response and change from parent study baseline in DAS28-CRP, at OLE study baseline and weeks 4, 24, 48, and 70. A total of 494 (93.9%) subjects completed the parent study; of these, 467 subjects who completed the week 26 visit were enrolled and 466 received treatment in the OLE study.
Clinical Efficacy
At baseline, the overall ACR20 response rate was 73.3%, which was sustained at subsequent weeks (Fig. 7) [19]. The mean change from baseline of the parent study was − 2.25 at baseline of the extension study. The mean change from baseline in DAS28-CRP of the parent study remained unchanged throughout the extension study. Also, the proportion of ACR20 responders was similar in the patient cohort that continued on ABP 501 and those who had transitioned from adalimumab RP to ABP 501. The efficacy, as assessed by ACR20 and DAS28-CRP, was maintained throughout the extension period and was similar among those patients who continued on ABP 501 and those who had transitioned from adalimumab RP to ABP 501.
OLE trial data in moderate to severe RA: percentage of patients achieving ACR20 [19]
Safety
Overall, 63.7% of all patients experienced ≥ 1 TEAE during the extension study; however, the incidence rates of TEAEs were similar in the group that continued ABP 501 from the parent study to the OLE study (ABP 501/ABP 501, 62.4%) and in those that transitioned from adalimumab RP to ABP 501 (adalimumab RP/ABP 501, 65.0%) [19]. Consistent with the parent study, common TEAEs that occurred in ≥ 5% of overall patients included nasopharyngitis (9.2%), upper respiratory tract infection (8.6%), bronchitis (6.4%), pharyngitis (4.1%) and urinary tract infection (3.6%). Throughout the study, a total of 59 SAEs in 46 patients (9.9%) were reported, with similar rates in the adalimumab RP/ABP 501 (8.9%) and ABP 501/ABP 501 (10.9%) cohorts. SAEs reported for more than one patient were osteoarthritis (n = 5), RA (n = 2), myocardial infarction (n = 2), and cataract (n = 3). Common AEs of interest during the study were infections, malignancies, hypersensitivity, hematological reactions, and liver enzyme elevations (Table 2). The most common infection was nasopharyngitis (adalimumab RP/ABP 501: 10.5%, ABP 501/ABP 501: 7.9%); other infections included upper respiratory tract infection, bronchitis, pharyngitis, urinary tract infection, sinusitis, oral herpes, tonsillitis, laryngitis, and pneumonia.
Immunogenicity in All Three Studies
Immunogenicity assessments were carried out in all three clinical studies. The development of binding and neutralizing antibodies was similar between ABP 501 and adalimumab RP in both the PsO and RA patient populations; these data are presented in Table 3 [15, 16, 18, 19]. Additionally, in the PsO study, despite the single transition switch from adalimumab RP to ABP 501 at week 16, the proportion of patients that developed binding and neutralizing antibodies was generally similar across groups over the entire 52 weeks of treatment, i.e., from baseline to study end (Table 3) [16]. Further, in the OLE study which included patients who had transitioned from adalimumab to ABP 501, the development of binding and neutralizing antibodies was also similar.
In the phase 3 RA study, the incidence rates of binding antibodies and neutralizing antibodies were similar between the two treatment groups throughout the study (Table 3) [18]. In the OLE study, relative to the OLE baseline, a similar proportion of patients were positive for binding antibodies and neutralizing antibodies, across the treatment groups anytime throughout the study (Table 3) [18]. Although the incidence rates of binding and neutralizing ADAs increased over time from the parent study baseline, the rates were maintained throughout the OLE study, with no differences in the adalimumab RP/ABP 501 and ABP 501/ABP 501 groups. Overall, no immunogenicity-related concerns were observed with ABP 501 during the 2 years of combined treatment [20].
It is important to note that, in the three clinical studies, a highly sensitive and drug-tolerant electrochemiluminescence (ECL) assay was used to assess ADA status. This may explain the higher rates of ADAs in these studies compared with those reported for adalimumab RP in the original studies. Of note, the rates were higher for both ABP 501 and adalimumab RP. The sensitivity of the ECL ADA binding assay was < 25 ng/mL in the presence of 25 μg/mL of the drug, with detection of 25 ng/mL ADAs [13, 21]. The ADA status in historical pivotal trials of adalimumab RP had been determined by ELISA assay, which is a less-sensitive assay that does not permit detection of ADAs in the presence of the drug [22]. The same ECL assay was used in all clinical studies of ABP 501 (phase 1, phase 3 and OLE) for the determination of ADAs and neutralizing antibodies.
Injection-Site Reactions and Injection-Site Pain Assessments in the Two Phase 3 Studies
An exploratory objective of both phase 3 studies was to assess patient perception of injection-site pain and injection-site reactions. Assessments were carried out within 5 min of injection at baseline, and weeks 4, 8, and 12, and measured using the validated 100-mm horizontal visual analog scale questionnaire [23, 24]. In the phase 3 study of patients with plaque PsO, the perception of pain was lower in the ABP 501 group (range 3.3–4.5 mm) compared with the adalimumab RP group (range 12.4–19.3 mm) at each time point including baseline. Overall, mean pain scores decreased over time in both treatment groups, from 4.5 mm at baseline to 3.3 mm at week 12 in the ABP 501 group and from 19.3 mm at baseline to 12.4 mm at week 12 in the adalimumab RP group. Similarly, injection-site reactions were lower in the ABP 501 group compared with the adalimumab RP group; adverse events in the ABP 501 group included injection-site hematoma, injection-site reaction, and injection-site erythema.
Similar to the findings of the PsO assessment, the perception of pain was lower in the ABP 501 group (range 10.0–10.7 mm) compared with the adalimumab RP group (range 16.1–21.4 mm) in the RA study [23, 24]. The mean pain scores remained nearly the same from baseline to week 12 in the ABP 501 group; however, the scores decreased over time in the adalimumab RP group, from 21.4 mm at baseline to 16.1 mm at week 12. Injection-site reactions were also lower in the ABP 501 group compared with the adalimumab RP group. The relevant events in the ABP 501 group included injection-site reaction and injection-site erythema, whereas those in the adalimumab RP group included injection-site erythema and injection-site reaction, injection-site pruritus, injection-site eczema, injection-site reaction hematoma, injection-site hypersensitivity, and injection-site pain; the event of injection-site eczema led to discontinuation of adalimumab RP.
The adalimumab RP used in the clinical studies contained citrate which has been linked to injection-site pain [25]. Therefore, the reduced perception of pain and injection-site reactions observed in the ABP 501 group may be attributed to the absence of citrate in the ABP 501 formulation. Potentially, the absence of injection site pain could result in increased compliance with ABP 501 treatment [25].
Overall Clinical Summary
The clinical confirmation component of the TOE for demonstration of ABP 501 similarity to adalimumab RP included a phase 1 PK equivalence study and two comparative phase 3 pivotal studies that evaluated the efficacy, safety, and immunogenicity of ABP 501 and adalimumab RP in two sensitive patient populations, PsO and RA.
In both phase 3 pivotal studies in PsO and RA, clinical efficacy of ABP 501 was demonstrated to be similar to the adalimumab RP. The OLE results further supported the clinical similarity between ABP 501 and adalimumab RP in RA by demonstrating that the efficacy was maintained over 72 weeks. Moreover, efficacy was similar among patients who continued on ABP 501 and those who had transitioned from adalimumab RP to ABP 501.
In terms of safety, there were no clinically meaningful differences in AEs or laboratory abnormalities in the two phase 3 pivotal studies, and no new safety signals emerged in the OLE study, supporting the long-term safety of ABP 501 for at least 2 years. Reports of hypersensitivity were also similar between the treatment arms. Malignancies occurred at a similar rate in both treatment arms and disease settings.
Immunogenicity was also shown to be similar between ABP 501 and adalimumab RP in the two comparative phase 3 studies as well as in the OLE study. Transition from the RP to ABP 501 did not impact immunogenicity, with similar rates of antibody formation in the ABP 501/ABP 501 and adalimumab RP/ABP 501 cohorts.
Extrapolation
The concept of extrapolation is unique to biosimilars and refers to seeking approval for biosimilar use in indications for which the adalimumab RP is approved without having to perform clinical trials in each of those indications [5, 6]. The scientific justification for extrapolation is based on the establishment of biosimilarity based on the TOE generated during development of the biosimilar program, including analytical, functional, non-clinical, and clinical data. Then, the mechanism of action, safety, immunogenicity, PK and evaluation of any other clinical risks for the RP are evaluated across the approved indications to determine if there are any differences that could preclude extrapolation. If none are identified, then extrapolation is supported based on a scientific justification of the above points and is predicated upon a determination of biosimilarity to the RP.
As discussed in this review, ABP 501 is similar to adalimumab RP based on a comprehensive analytical and functional assessment and shows similar non-clinical performance. In clinical evaluations, ABP 501 exhibits similar PK profiles in healthy subjects as well as in subjects with RA and PsO. Additionally, ABP 501 shows similar efficacy, safety, and immunogenicity in both RA and PsO patient populations. Of note, immunogenicity was similar in patients taking MTX as a concomitant medication (RA study) as well as those not on immunosuppressive medications (PsO study). Taken together, these data support the conclusion of biosimilarity. Without identified differences in the mechanism of action, PK, safety, or immunogenicity of the RP across the approved indications of adalimumab, extrapolation to all approved indications of the adalimumab RP (i.e., AS, PsA, UC, and CD) is also supported. In fact, ABP 501 received approval for use in all indications approved for the adalimumab RP, except those protected by regulatory exclusivity.
Overall conclusions
The regulatory pathway for demonstration of similarity between a proposed biosimilar and its RP requires a systematic stepwise approach, in which evidence of similarity of structure, function, PK (and PD where appropriate), clinical efficacy, safety, and immunogenicity is built by methodical accumulation of scientifically robust comparative data between the proposed biosimilar and its RP. The sum of these results, referred to as the TOE in support of biosimilarity, forms the basis of approval of a biosimilar. In accordance with this TOE approach, all evaluations demonstrated that ABP 501 and its adalimumab RP are highly similar (including 2 years of clinical data in RA and 1 year in PsO). Additionally, a single switch from adalimumab RP to ABP 501 was safe and without loss of efficacy. The results presented here led to the approval of ABP 501 as the first biosimilar to adalimumab.
References
Pimentel-Muinos FX, Seed B. Regulated commitment of TNF receptor signaling: a molecular switch for death or activation. Immunity. 1999;11(6):783–93.
Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology. 2010;49(7):1215–28 (Oxford).
AMJEVITA (adalimumab-atto) injection for subcutaneous use [prescribing information]. Thousand Oaks, CA: Amgen Inc, 2016.
AMGEVITA (adalimumab) injection for subcutaneous use [summary of product characteristics]. Breda, The Netherlands: Amgen Europe, 2017.
Food and Drug Administration. Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf. Accessed 3 Aug 2018.
European Medicines Agency. Questions and answers on biosimilar medicines (similar biological medicinal products). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf. Accessed 3 Aug 2018.
World Health Organization. Guidelines on evaluation of Similar Biotherapeutic Products (SBPs). October 2009. http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf. Accessed 3 Aug 2018.
Markus R, Liu J, Ramchandani M, Landa D, Born T, Kaur P. Developing the totality of evidence for biosimilars: regulatory considerations and building confidence for the healthcare community. BioDrugs. 2017;31:175–87.
Liu J, Eris T, Li C, Cao S, Kuhns S. Assessing analytical similarity of proposed Amgen biosimilar ABP 501 to adalimumab. BioDrugs. 2016;30:321–38.
Sivendran R, Ramirez J, Ramchandani M, Liu J. Scientific and statistical considerations in evaluating the analytical similarity of ABP 501 to adalimumab. Immunotherapy. 2018;10(11):1011–1021.
Velayudhan J, Chen YF, Rohrbach A, et al. Demonstration of functional similarity of proposed biosimilar ABP 501 to Adalimumab. BioDrugs. 2016;30:339–51.
McBride H, Kuhns S, Foltz I, Sweet H, Kaur P. ABP 501: matching the critical functions of Adalimumab. Gastroenterology. 2017;152(5):S765.
Kaur P, Chow V, Zhang N, Moxness M, Kaliyaperumal A, Markus R. A randomised, single-blind, single-dose, three-arm, parallel-group study in healthy subjects to demonstrate pharmacokinetic equivalence of ABP 501 and adalimumab. Ann Rheum Dis. 2017;76(3):526–33.
Doshi S, Krishnan E, Wang H, Zhang N, Chow V. Establishing PK equivalence between Adalimumab and ABP 501 in the presence of antidrug antibodies using population PK modeling. J Crohns Colitis. 2018;12(supp 1):S471.
Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. J Am Acad Dermatol. 2017;76:1093–102.
Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;177(6):1562–74.
Krishnan E, Wang H, Zhang N. Psoriatic arthritis patient experience with ABP 501 and Adalimumab. In: Presented at European Academy of Dermatology and Venereology (EADV); September 12–16, 2018; Paris, France.
Cohen S, Genovese MC, Choy E, et al. Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: a randomised, double-blind, phase III equivalence study. Ann Rheum Dis. 2017;76:1679–87.
Cohen S, Pablos JL, Pavelka K, et al. An open label extension study in rheumatoid arthritis patients to demonstrate long-term safety and efficacy of ABP 501. Arthritis Res Ther. 2019;21:84 .
Krishnan E, Mytych D, Zhang N, et al. Immunogenicity associated with a transition from adalimumab reference product to ABP 501 in patients with rheumatoid arthritis. Ann Rheum Dis. 2018;77:318.
Moxness M, Tatarewicz S, Weeraratne D, et al. Immunogenicity testing by electrochemiluminescent detection for antibodies directed against therapeutic human monoclonal antibodies. Clin Chem. 2005;51(10):1983–5.
Song S, Yang L, Trepicchio WL, Wyant T. Understanding the supersensitive anti-drug antibody assay: unexpected high anti-drug antibody incidence and its clinical relevance. J Immunol Res. 2016;2016:3072586.
Kaur P, Cohen S, Papp K, et al. ABP 501 (AMJEVITA™) Biosimilar to Adalimumab: demonstration of value with lower perception of injection site pain. In: Presented at the Academy of Managed Care Pharmacy Annual Meeting; March 27–30, 2017; Denver, CO.
Krishnan E, Zhang N, Wang H. Injection site reactions and injection site pain for the Adalimumab biosimilar ABP 501: results from two double-blind randomized controlled studies. In: Poster presented at European Crohn’s and Colitis Organization (ECCO) Congress; February 14–17, 2018; Vienna, Austria.
Laursen T, Hansen B, Fisker S. Pain perception after subcutaneous injections of media containing different buffers. Basic Clin Pharmacol Toxicol. 2006;98(2):218–21.
Acknowledgements
The authors wish to thank Teresa Born, Scott Kuhns, and Jyoti Velayudhan for their contributions to pre-clinical evaluations.
Funding
Sponsorship of ABP 501 studies described in this review, the article processing charges and the Open Access fee were funded by Amgen Inc., Thousand Oaks, CA, USA. All authors had full access to the articles reviewed in this manuscript and take complete responsibility for the integrity and accuracy of this manuscript.
Editorial Assistance
Editing assistance was funded by Amgen Inc. and provided by Sabby Muneer, PhD, from Innovation Communications Group, Inc., New York, NY, USA, under the guidance of Monica Ramchandani, PhD, Amgen Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Richard Markus is an Amgen employee and stockholder. Helen J. McBride is an Amgen employee and stockholder. Monica Ramchandani is an Amgen employee and stockholder. Vincent Chow is an Amgen employee and stockholder. Jennifer Liu is an Amgen employee and stockholder. Dan Mytych is an Amgen employee and stockholder. Gary Fanjiang is an Amgen employee and stockholder.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current review.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to: https://doi.org/10.6084/m9.figshare.7998434.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Markus, R., McBride, H.J., Ramchandani, M. et al. A Review of the Totality of Evidence Supporting the Development of the First Adalimumab Biosimilar ABP 501. Adv Ther 36, 1833–1850 (2019). https://doi.org/10.1007/s12325-019-00979-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-00979-6