Abstract
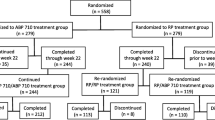
BCD-055 is a biosimilar of innovator infliximab (IFX). Here we present the 54-week results from phase 3 clinical study in patients with rheumatoid arthritis (RA). The aim of this study was to demonstrate the equivalent efficacy and safety of BCD-055 and IFX in patients with active rheumatoid arthritis. 426 adults with active RA were enrolled. Patients were randomized into 2 study arms in 2:1 ratio to receive BCD-055 or IFX innovator in dose of 3 mg/kg at week 0, 2, 6 and then every 8 weeks up to week 54. Primary efficacy endpoint was the rate of American College of Rheumatology (ACR) 20 response at week 14. The equivalence margin was set as 15%. Immunogenicity and safety were also assessed. Rate of ACR20 at week 14 in PP (Per-Protocol) population was 71.2% in BCD-055 group and 67.9% in IFX group. Difference in ACR20 rates between groups was 3.2% with 95% CI [− 7.0%; 13.5%] (р = 0.587). Throughout 54-week study period, both groups were characterized by similar rates of ACR20/50/70 response at all timepoints without significant differences (p > 0.05). The rates of adverse events (AE) were similar in groups (74.64% in BCD-055 arm vs 66.67% in IFX arm, p = 0.111). Antibodies to infliximab were detected in 28.46% patients for BCD-055 arm and 26.56% for IFX arm (p = 0.786). BCD-055 and IFX were comparable in efficacy (including radiographic progression), safety and immunogenicity throughout the 54-week study.
Trial registration ClinicalTrials.gov ID, number NCT02762838.



Similar content being viewed by others
References
Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388:2023–2038. https://doi.org/10.1016/s0140-6736(16)30173-8
Smolen JS, Aletaha D et al (2018) Rheumatoid arthritis. Nat Rev Dis Prim 4:18001. https://doi.org/10.1038/nrdp.2018.1
Mclnnes IB, Schett G (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7(6):429–442
Elliott M, Maini R, Feldman M et al (1993) Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor α. Arthritis Rheum 36:1681–1690
Maini R, Clair EW, Breedveld F, ATTRACT Study Group et al (1999) Infliximab (chimeric anti-tumor necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet 354(9194):1932–1939
World Health Organization, Expert Committee on Biological Standardization (2009) Guidelines on evaluation of similar biotherapeutic products (SBPs). http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf. Accessed 11 Dec 2018
Generics and Biosimilars Initiative (2016) Biosimilars approved in Europe. http://www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe. Accessed 28 Aug 2018
Denisov L, Gordeev I, Mazurov V et al (2017) FRI0208 Comparison of efficacy, safety and pharmacokinetics of infliximab biosimilar (BCD-055) and innovator infliximab. Ann Rheum Dis 76:560–561
Ezard C, Kumari R, Willot R et al (2012) What is meant by active disease in the NICE recommendation on use of combination therapy in early RA? Rheumatology 51(5):947–948
van der Heijde D (2000) How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 27:261–263
Steinbrocker O, Traeger CH, Batter-Mann RC (1949) Therapeutic criteria in rheumatoid arthritis. JAMA 140:659–662
Glueck DH (2008) Sample size calculations in clinical research. In: Chow SC, Shao J, Wang H (eds) Biometrics, vol 64, 2nd edn. CRC Press, Boca Raton, pp 1307–1308. https://doi.org/10.1111/j.1541-0420.2008.01138_10.x
Clair EWS, Van der Heijde D, Smolen JS et al (2004) Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 50(11):3432–3443
Yoo DH, Hrycaj P, Miranda P et al (2013) A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 72:1613–1620
Park W, Hrycaj P, Jeka S et al (2013) A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis 72:1605–1612
Choe JY, Prodanovic N, Niebrzydowski J et al (2017) A randomised, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 76(1):58–64
Cohen SB, Alten R, Kameda H et al (2018) A randomized controlled trial comparing PF-06438179/GP1111 (an infliximab biosimilar) and infliximab reference product for treatment of moderate to severe active rheumatoid arthritis despite methotrexate therapy. Arthritis Res Ther. 20(1):155. https://doi.org/10.1186/s13075-018-1646-4
Yoo DH, Racewicz A, Brzezicki J et al (2016) A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 18:82. https://doi.org/10.1186/s13075-016-0981-6
Smolen JS, Jung-Yoon Choe, Prodanovic N et al (2017) Comparing biosimilar SB2 with reference infliximab after 54 weeks of a double-blind trial: clinical, structural and safety results. Rheumatology 56:1771–1779. https://doi.org/10.1093/rheumatology/kex254
Dixon WG, Hyrich KL, Watson KD, on behalf of the BSR Biologics Register et al (2010) Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis 69:522–528
Wolfe F, Michaud K (2007) Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum 56:2886–2895
Askling J, van Vollenhoven RF, Granath F et al (2009) Cancer risk in patients with rheumatoid arthritis treated with anti–tumor necrosis factor α therapies: does the risk change with the time since start of treatment? Arthritis Rheum 60:3180–3189
Aikawa NE, de Carvalho JF, Almeida Silva CA, Bonfa E (2010) Immunogenicity of anti-TNF-alpha agents in autoimmune diseases. Clin Rev Allergy Immunol 38(2–3):82–89
European Medicines Agency (2014) [Internet] Guideline on similar biological medicinal products. London, UK: Committee for Medicinal Products for Human Use (CHMP), European Medicines Agency; 2014. [updated October 23, 2014]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf. Accessed 23 Apr 2019
United States Food and Drug Administration (2015) [Internet] Scientific considerations in demonstrating biosimilarity to a reference product: guidance for industry. Silver Spring, MD: US Department of Health and Human Services, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER); 2015. [updated April 2015]. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm291128.pdf. Accessed 23 Apr 2019
Vulto AG, Jaquez OA (2017) The process defines the product: what really matters in biosimilar design and production? Rheumatology 56(suppl_4):iv14–iv29. https://doi.org/10.1093/rheumatology/kex278
Smolen JS, Landewe R, Breedveld FC et al (2017) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 76:960–977
Yoo DH, Prodanovic N, Jaworski J et al (2017) Efficacy and safety of CT-P13 (infliximab biosimilar) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis 76:355–363
Park W, Yoo DH, Miranda P et al (2017) Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance CT-P13 in ankylosing spondylitis. 102-week data from the PLANETAS extension study. Ann Rheum Dis 76:346–354
Goll GL, Jørgensen KK, Sexton J, Olsen IC et al (2019) Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: open-label extension of the NOR-SWITCH trial. J Intern Med. https://doi.org/10.1111/joim.12880
Acknowledgements
The authors appreciate Nikita Zolkin and Ivan Kuryshev (JSC BIOCAD, Russia) for statistical analysis, and support in manuscript development. Employees of JSC BIOCAD, the study sponsor, designed the study in collaboration with the academic authors, conducted the study, collected the data, performed statistical analyses, and were involved in the writing of the study manuscript. Contributing centers (principal investigators)—Russia: Olga V. Antipova, Anna N. Galustyan, Ivan G. Gordeev, Antonina F. Davidova, Natalya A. Eremina, Alexander A. Kastanyan, Larisa A. Knayzeva, Tatyana V. Kropotina, Irina M. Marusenko, Irina M. Patrikeeva, Tatyana V. Plaksina, Tatyana V. Povarova, Tatyana G. Pokrovskaya, Olga V. Reshetko, Elena A. Smolyarchuk, Irina A. Shumichina, Elena V. Zemerova, Elena V. Zonova, Eduard A. Ponomarev, Svetlana A. Smakotina, Marianna S. Petrova, Galina A. Chumakova, Larisa V. Eliseeva, Maria I. Skalinskaya. Belarus: Igor E. Adzericho, Sergey I. Pimanov, Andrey M. Pristom, Elena V. Kunder, Nikolay F. Soroka. India: Reema Kashiva, Ajit Bapurao Nalawade, Liyakat Ali Gauri, Vishnu Sharma, Syamasis Bandyopadhyay, Bindumathi P. L.
Funding
This study was sponsored by JSC BIOCAD.
Author information
Authors and Affiliations
Contributions
In accordance with ICMJE criteria, all authors were involved in writing and drafting the article or revising it critically for important intellectual content. All authors approved the final version to be submitted for publication and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
Alexander M. Lila, Vadim I. Mazurov, Lev N. Denisov, Olga B. Nesmeyanova, Elena P. Ilivanova declare that they have no conflict of interest. Anna V. Eremeeva, Julia V. Usacheva, Ekaterina V. Chernyaeva, Ekaterina A. Dokukina and Roman A. Ivanov Employees of JCS BIOCAD.
Ethical approval
All patients were informed, and their informed consents were obtained prior to the study. The information on all Ethics Committees and approval dates is presented in Supplementary Material B.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lila, A.M., Mazurov, V.I., Denisov, L.N. et al. A phase III study of BCD-055 compared with innovator infliximab in patients with active rheumatoid arthritis: 54-week results from the LIRA study. Rheumatol Int 39, 1537–1546 (2019). https://doi.org/10.1007/s00296-019-04359-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04359-9