Abstract
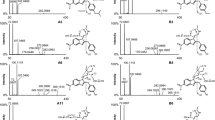
LJ-2698, a highly potent human A3 adenosine receptor antagonist with nucleoside structure, was designed to have a minimal species dependence. For further pre-clinical studies, analytical method for the detection of LJ-2698 in rat plasma was developed by liquid chromatography-tandem mass. Plasma samples were processed by protein precipitation method with acetonitrile, using losartan as the internal standard (IS). Chromatographic separation was carried out using a Kinetex C18 column (100 × 4.6 mm; 100 Å; 2.6 μ) with acetonitrile/water with 0.2% (v/v) formic acid (65:35, v/v) in the isocratic mode at a flow rate of 0.4 mL/min. Mass spectrometric detection in multiple reaction monitoring mode was performed with positive electrospray ionization. The mass transitions of LJ-2698 and IS were m/z 412.3 → 294.1 and m/z 423.1 → 207.2, respectively. The calibration curves were linear in the range 5.00–5000 ng/mL (r 2 ≥ 0.998). The lower limit of quantification was established as 5.00 ng/mL. Within- and between-run precisions were <7.01%, as relative standard deviation; and accuracies were in the range 3.37–3.64%, as relative error. The validated method was successfully applied to its pharmacokinetic evaluation after intravenous and oral administration in rats, and the dose-dependent pharmacokinetic behavior of LJ-2698 was elucidated for the first time.




Similar content being viewed by others
References
Appel S, RaA Mathôt, Langemeijer MWE, Ijzerman AP, Danhof M (1995) Modelling of the pharmacodynamic interaction of an A1 adenosine receptor agonist and antagonist in vivo: N6-cyclopentyladenosine and 8-cyclopentyltheophylline. Br J Pharmacol 115:1253–1259
Baraldi PG, Tabrizi MA, Romagnoli R, Fruttarolo F, Merighi S, Varani K, Gessi S, Borea PA (2005) Pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine ligands, new tools to characterize A3 adenosine receptors in human tumor cell lines. Curr Med Chem 12:1319–1329
Borea PA, Varani K, Vincenzi F, Baraldi PG, Tabrizi MA, Merighi S, Gessi S (2015) The A3 adenosine receptor: history and perspectives. Pharmacol Rev 67:74–102
Chen GJ, Harvey BK, Shen H, Chou J, Victor A, Wang Y (2006) Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res 84:1848–1855
Cruz-Monteagudo M, Cordeiro MN, Teijeira M, Gonzalez MP, Borges F (2010) Multidimensional drug design: simultaneous analysis of binding and relative efficacy profiles of N(6)-substituted-4′-thioadenosines A3 adenosine receptor agonists. Chem Biol Drug Des 75:607–618
Fishman P, Bar-Yehuda S (2003) Pharmacology and therapeutic applications of A3 receptor subtype. Curr Top Med Chem 3:463–469
Food and Drug Administration (2013) Guidance for Industry: Bioanalytical Method Validation. U.S. Department of Health and Human Services. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm368107.pdf. Accessed 1 Jan 2016
Gao ZG, Joshi BV, Klutz AM, Kim SK, Lee HW, Kim HO, Jeong LS, Jacobson KA (2006) Conversion of A3 adenosine receptor agonists into selective antagonists by modification of the 5′-ribofuran-uronamide moiety. Bioorg Med Chem Lett 16:596–601
Gillespie TA, Winger BE (2011) Mass spectrometry for small molecule pharmaceutical product development: a review. Mass Spectrom Rev 30:479–490
Hope E, Mayorga SR, Robinson S, Goldberg L, Leveson JE, Marengo-Rowe AJ, Peerschke EIB (1991) Preparation of plasma for coagulation testing: evaluation of the StatSpin® high-speed centrifuge. Lab Med 22:190–193
Hou X, Kim HO, Alexander V, Kim K, Choi S, Park S-G, Lee JH, Yoo LS, Gao Z-G, Jacobson KA, Jeong LS (2010) Discovery of A new human A(2A) adenosine receptor agonist, truncated 2-hexynyl-4′-thioadenosine. ACS Med Chem Lett 1:516–520
Jacobson KA, Gao ZG (2006) Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5:247–264
Jeong LS, Jin DZ, Kim HO, Shin DH, Moon HR, Gunaga P, Chun MW, Kim YC, Melman N, Gao ZG, Jacobson KA (2003) N6-substituted D-4′-thioadenosine-5′-methyluronamides: potent and selective agonists at the human A3 adenosine receptor. J Med Chem 46:3775–3777
Jeong LS, Choe SA, Gunaga P, Kim HO, Lee HW, Lee SK, Tosh DK, Patel A, Palaniappan KK, Gao ZG, Jacobson KA, Moon HR (2007) Discovery of a new nucleoside template for human A3 adenosine receptor ligands: D-4′-thioadenosine derivatives without 4′-hydroxymethyl group as highly potent and selective antagonists. J Med Chem 50:3159–3162
Lee J-H, An T-G, Kim SJ, Shim W-S, Lee K-T (2015) Development of liquid chromatography tandem mass spectrometry method for determination of spironolactone in human plasma: application to a bioequivalence study of Daewon Spiracton tablet® (spironolactone 50 mg). J Pharm Investig 45:601–609
Lee J-Y, Kim S-B, Chun J, Song KH, Kim YS, Chung S-J, Cho H-J, Yoon I-S, Kim D-D (2016) High body clearance and low oral bioavailability of alantolactone, isolated from Inula helenium, in rats: extensive hepatic metabolism and low stability in gastrointestinal fluids. Biopharm Drug Dispos 37:156–167
Lin JH, deLuna FA, Ulm EH, Tocco DJ (1990) Species-dependent enantioselective plasma protein binding of MK-571, a potent leukotriene D4 antagonist. Drug Metab Dispos 18:484–487
Madi L, Bar-Yehuda S, Barer F, Ardon E, Ochaion A, Fishman P (2003) A3 adenosine receptor activation in melanoma cells: association between receptor fate and tumor growth inhibition. J Biol Chem 278:42121–42130
Mathôt RA, Van Schaick EA, Langemeijer MW, Soudijn W, Breimer DD, Ijzerman AP, Danhof M (1994) Pharmacokinetic-pharmacodynamic relationship of the cardiovascular effects of adenosine A1 receptor agonist N6-cyclopentyladenosine in the rat. J Pharmacol Exp Ther 268:616–624
Mulloy DP, Sharma AK, Fernandez LG, Zhao Y, Lau CL, Kron IL, Laubach VE (2013) Adenosine A3 receptor activation attenuates lung ischemia-reperfusion injury. Ann Thorac Surg 95:1762–1767
Nandi U, Dan S, Pal TK (2015) Development and validation of a liquid chromatography–mass spectrometry method for simultaneous determination of metoprolol and telmisartan in rat plasma and its application to pharmacokinetic study. J Pharm Investig 45:329–340
Olah ME, Stiles GL (2000) The role of receptor structure in determining adenosine receptor activity. Pharmacol Ther 85:55–75
Olah ME, Gallo-Rodriguez C, Jacobson KA, Stiles GL (1994) 125I-4-aminobenzyl-5′-N-methylcarboxamidoadenosine, a high affinity radioligand for the rat A3 adenosine receptor. Mol Pharmacol 45:978–982
Park SM (2008) Pharmacokinetics of adenosine Areceptor agonist, 2-chloro-N^(6)-(3-iodobenzyl)-4′-thioadenosine-5′-N-methyluronamide, Dissertation, Ewha Womans University
Press NJ, Keller TH, Tranter P, Beer D, Jones K, Faessler A, Heng R, Lewis C, Howe T, Gedeck P, Mazzoni L, Fozard JR (2004) New highly potent and selective adenosine A(3) receptor antagonists. Curr Top Med Chem 4:863–870
RaA Mathôt, Cleton A, Soudijn W, Ijzerman AP, Danhof M (1995) Pharmacokinetic modelling of the haemodynamic effects of the A2a adenosine receptor agonist CGS 21680C in conscious normotensive rats. Br J Pharmacol 114:761–768
Ramkumar V, Stiles GL, Beaven MA, Ali H (1993) The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem 268:16887–16890
Sachdeva S, Gupta M (2013) Adenosine and its receptors as therapeutic targets: an overview. Saudi Pharm J 21:245–253
Sajjadi FG, Takabayashi K, Foster AC, Domingo RC, Firestein GS (1996) Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. J Immunol 156:3435–3442
Siddiqi SM, Jacobson KA, Esker JL, Olah ME, Ji XD, Melman N, Tiwari KN, Secrist JA 3rd, Schneller SW, Cristalli G (1995) Search for new purine- and ribose-modified adenosine analogues as selective agonists and antagonists at adenosine receptors. J Med Chem 38:1174–1188
Tracey WR, Magee WP, Oleynek JJ, Hill RJ, Smith AH, Flynn DM, Knight DR (2003) Novel N6-substituted adenosine 5′-N-methyluronamides with high selectivity for human adenosine A3 receptors reduce ischemic myocardial injury. Am J Physiol Heart Circ Physiol 285:H2780–H2787
Tsai Y-J, Lin L-C, Tsai T-H (2010) Pharmacokinetics of adenosine and cordycepin, a bioactive constituent of cordyceps sinensis in rat. J Agric Food Chem 58:4638–4643
Van Eeckhaut A, Lanckmans K, Sarre S, Smolders I, Michotte Y (2009) Validation of bioanalytical LC–MS/MS assays: evaluation of matrix effects. J Chromatogr B Analyt Technol Biomed Life Sci 877:2198–2207
Van Ginneken CA, Russel FG (1989) Saturable pharmacokinetics in the renal excretion of drugs. Clin Pharmacokinet 16:38–54
Van Tilburg EW, Künzel JVFD, Ijzerman AP (2002) 2,5‘-Disubstituted adenosine derivatives: evaluation of selectivity and efficacy for the adenosine A1, A2A, and A3 receptor. J Med Chem 45:420–429
Volpini R, Costanzi S, Lambertucci C, Vittori S, Klotz K, Lorenzen A, Cristalli G (2001) Introduction of alkynyl chains on C-8 of adenosine led to very selective antagonists of the A(3) adenosine receptor. Bioorg Med Chem Lett 11:1931–1934
Yang H, Avila MY, Peterson-Yantorno K, Coca-Prados M, Stone RA, Jacobson KA, Civan MM (2005) The cross-species A3 adenosine-receptor antagonist MRS 1292 inhibits adenosine-triggered human nonpigmented ciliary epithelial cell fluid release and reduces mouse intraocular pressure. Curr Eye Res 30:747–754
Yoon IS, Choi MK, Kim JS, Shim CK, Chung SJ, Kim DD (2011) Pharmacokinetics and first-pass elimination of metoprolol in rats: contribution of intestinal first-pass extraction to low bioavailability of metoprolol. Xenobiotica 41:243–251
Acknowledgements
This work was supported by the Basic Study for Well Aging Project funded by the Seokchun Daewoong Foundation (2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest with this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, JY., Park, JH., Kim, KT. et al. Determination and validation of LJ-2698, a potent human A3 adenosine receptor antagonist, in rat plasma by liquid chromatography-tandem mass spectrometry and its application in pharmacokinetic study. Arch. Pharm. Res. 40, 952–961 (2017). https://doi.org/10.1007/s12272-017-0935-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0935-9