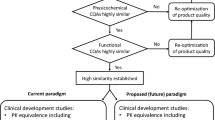
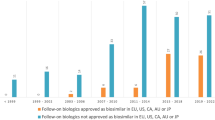
Abstract
Purpose of Review
This review provides an update on the evolution of, manufacturing of, and regulations for biosimilars in Asian countries.
Recent Findings
The use of biologics revolutionized the treatment of various diseases. However, the high cost of biologics remained unaffordable for most Asian patients and increases the financial burden of Asian governments. The development of biosimilars provides the best solution for this predicament. A great boom of biosimilars is developing in Asia. Hundreds of biopharmaceutical companies have established their manufacturing facilities and provide affordable products for Asian patients. Regulation guidelines and international harmonization are evolving.
Summary
Better manufacturing quality and post-market pharmacovigilance are needed in Asia despite the rapid evolution of biosimilar development.

Similar content being viewed by others
References
Recently published papers of particular interest have been highlighted as: • Of importance
Kim EY, Moudgil KD. Immunomodulation of autoimmune arthritis by pro-inflammatory cytokines. Cytokine. 2017; doi:10.1016/j.cyto.2017.04.012.
Schinnerling K, Aguillón JC, Catalán D, Soto L. The role of interleukin-6 signaling and its therapeutic blockage in skewing the T cell balance in rheumatoid arthritis. Clin Exp Immunol. 2017; doi:10.1111/cei.12966.
Scheinberg MA, Azevedo VF. Biosimilars in rheumatology: perspective and concerns. Rheumatology (Oxford). 2014;53(3):389–90.
Top 50 pharmaceutical products by global sales. http://www.pmlive.com/top_pharma_list/Top_50_pharmaceutical_products_by_global_sales (accessed 22/Apr/2017).
The brave new world of biosimilars. https://www.futurereadysingapore.com/2017/the-brave-new-world-of-biosimilars.html (accessed 22/Apr/2017).
• Bas TG, Oliu Castillo C. Biosimilars in developed and developing east and Southeast Asian countries: Japan, South Korea, and Malaysia—overview, evolution, and regulations assessment. Biomed Res Int. 2016(5910403):12. This article describes the unmet need for better treatment in Asian countries, and it further illustrates the evolution and regulation of biosimialr manufacturing industry in these countries
• Al-Sabbagh A, Olech E, McClellan JE, Kirchhoff CF. Development of biosimilars. Semin arthritis rheum. 2016 45(5 Suppl):S11–S18. This article illustrates the manufacturing process and quality control for biosimialr products, the comprehensive description details and the tight control of each manufacturing process for biosimilar products.
Blandizzi C, Meroni PL, Lapadula G. Comparing originator biologics and biosimilars: a review of the relevant issues. ClinTher. 2017; doi:10.1016/j.clinthera.2017.03.014.
Markenson J, Alvarez DF, Jacobs I, Kirchhoff C. A practical guide about biosimilar data for health care providers treating inflammatory diseases. Biologics. 2017;11:13–21.
Clinicaltrials.gov. http://clinicaltrials.gov (accessed 22/Apr/2017).
http://www.pharmachinaonline.com (Accessed 22/Apr/2017).
China FDA. http://eng.sfda.gov.cn/WS03/CL0755/search.html?columnid=WID|CTITLE|CONTENT&relation=MUST|SHOULD|SHOULD&tableName=Region&colNum=3&qryNum=3&curPage=1&qryidstr=wid|qry1|qry1&qryValue=ws03|biosimilars|biosimilars (accessed 22/Apr/2017).
Christl LA, Woodcock J, Kozlowski S. Biosimilars: the US regulatory framework. Annu Rev Med. 2017;68:243–54.
EMAguideline on similar biological medicinal products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf (accessed 25/Apr/2017).
• Declerck P, Danesi R, Petersel D, Jacobs I. The language of biosimilars: Clarification, definitions, and regulatory aspects. Drugs. 2017;77:671–7. This paper provides clear definition of terms used for the description of biologic products
Rathore AS. Biosimilars in India. J Proteome. 2015;127:71–2.
Guidelines on similar biologics: regulatory requirements for marketing authorization in India. http://www.cdsco.nic.in/writereaddata/Bio%20Similar%20Guideline.pdf (accessed 25/Apr/2017).
Biosimilars approved in Japan Oct 2016 update. http://gabionline.net/Biosimilars/General/Biosimilars-approved-in-Japan (accessed 25/Apr/2017).
Arato T. Japanese regulation of biosimilar products: past experience and current challenges. Br J ClinPharmacol. 2016;82(1):30–40.
Naqasaki M, Ando Y. Clinical development and trial design of biosimilar products: a Japanese perspective. J Biopharm J Biopharm Stat. 2014;24(6):1165–72.
Derbyshire M. Biosimilar development and regulation in Japan. Generics and Biosimilars Initiative Journal (GaBI Journal). 2013;2(4):207–8.
Biosimilars approved in South Korea 2016 update. http://www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-South-Korea (accessed 25/Apr/2017).
Korean guidelines on the evaluation of biosimilar products. http://www.kfda.go.kr/index.kfda?mid-226&pageNo-1&seq-388&cmd-v; July 2009 (accessed 26/Apr/2017).
Suh SK, Park Y. Regulatory guideline for biosimilar products in Korea. Biologicals. 2011;39:336–8.
https://www.bloomberg.com/research/stocks/private/snapshot.asp?privcapId=241607919 (accessed 26/Apr/2017).
Taiwan FDA. file:///C:/Documents%20and%20Settings/station/My%20Documents/Downloads/ (accessed 26/Apr/2017).
Poh J, Tam KT. Registration of similar biological products—Singapore’s approach. Biologicals. 2011;39(5):343–5.
National Pharmaceutical Control Bureau. Ministry of Health. Malaysia, “Drug registration guidance document (DRGA),” 2013, http://rr.mpc.gov.my/data/lic-legal-2013-12-24-06-40-33.pdf .
http://www.biotechcorp.com.my/press-release/viropro-andoncobiologics-sign-emerging-markets-biosimilars-deal/ (accessed 18/ Apr/2017).
Kadam V, Bagde S, Karpe M, Kadam V. A comprehensive overview on biosimialrs. Curr Protein Pept Sci. 2016;17(8):756–61.
Stevenson JG, Green L, pharmacovigilance, and patient safety: It’s all in the name.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by author.
Additional information
This article is part of the Topical Collection on Biosimilars
Rights and permissions
About this article
Cite this article
Tsai, WC. Update on Biosimilars in Asia. Curr Rheumatol Rep 19, 47 (2017). https://doi.org/10.1007/s11926-017-0677-1
Published:
DOI: https://doi.org/10.1007/s11926-017-0677-1