Abstract
Purpose
We evaluated 18F-2-fluoro-2-deoxyglucose positron emission tomography/computed tomography (FDG PET/CT) results as outcome predictors for patients with metastatic renal cell carcinoma (RCC) treated by everolimus (EVL), an inhibitor of mammalian target of rapamycin.
Methods
We retrospectively reviewed 30 patients who were treated with EVL for metastatic RCC between May 2010 and March 2015, by evaluating their FDG PET/CT result before and 1 month after starting EVL treatment. We examined the relationships between each patient’s maximum standardized uptake value (max SUVmax) assessed by FDG PET/CT on progression-free survival (PFS) and overall survival (OS).
Results
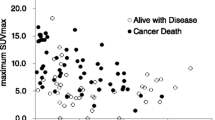
Median PFS for all 30 patients was 3.77 months (range 0.72–24.56 months) and median OS after EVL treatment of all 30 patients was 11.67 months (range 1.0–62.98 months). Enrolled patients were divided into two groups by max SUVmax prior to EVL (median = 7.6) and at 1 month after EVL treatment (median = 5.7). PFS were significantly shorter in higher max SUVmax prior to EVL (<7.6, PFS 7.8 vs 3.5 months, log-rank P = 0.017) and at 1 month after EVL (<5.7, PFS 10.6 vs 2.7 months, log-rank P = 0.002) than lower max SUVmax. OS were also significantly shorter in higher max SUVmax prior to EVL (<7.6, OS 18.1 vs 7.5 months, log-rank P = 0.010) and at 1 month after EVL (<5.7, OS 17.2 vs 7.5 months, log-rank P = 0.009) than lower max SUVmax. Multivariate Cox hazard regression analysis indicated that max SUVmax at 1 month after EVL is an independent predictor of both PFS and OS in patients treated with EVL although univariate regression analysis showed max SUVmax before EVL is a possible predictor.
Conclusions
Max SUVmax assessed by FDG PET/CT prior to EVL and at 1 month after EVL treatment can accurately predict PFS and can guide decisions on whether to continue or change treatments for patients with EVL-treated RCC who suffer from adverse events.




Similar content being viewed by others
References
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149:274–293
Sengupta S, Peterson TR, Sabatini DM (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40:310–322
Motzer RJ, Escudier B, Oudard S et al (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372:449–456
European Association of Urology (2014) European Association of Urology guidelines 2014 edition. http://www.uroweb.org/guidelines/online-guidelines/
National Comprehensive Cancer Network (2015) NCCN Clinical Practice Guideline in Oncology: Kidney Cancer V. 1. 2015. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site
Majhail NS, Urbain JL, Albani JM et al (2003) F-18 fluorodeoxyglucose positron emission tomography in the evaluation of distant metastases from renal cell carcinoma. J Clin Oncol 21:3995–4000
Park JW, Jo MK, Lee HM (2009) Significance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography for the postoperative surveillance of advanced renal cell carcinoma. BJU Int 103:615–619
Namura K, Minamimoto R, Yao M et al (2010) Impact of maximum standardized uptake value (SUVmax) evaluated by 18-Fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (18F-FDG-PET/CT) on survival for patients with advanced renal cell carcinoma: a preliminary report. BMC Cancer 10:667
Nakaigawa N, Kondo K, Tateishi U et al (2016) FDG PET/CT as a prognostic biomarker in the era of molecular-targeting therapies: max SUVmax predicts survival of patients with advanced renal cell carcinoma. BMC Cancer 16:67
Minamimoto R, Nakaigawa N, Tateishi U et al (2010) Evaluation of response to multikinase inhibitor in metastatic renal cell carcinoma by FDG PET/contrast-enhanced CT. Clin Nucl Med 35:918–923
Ueno D, Yao M, Tateishi U et al (2012) Early assessment by FDG-PET/CT of patients with advanced renal cell carcinoma treated with tyrosine kinase inhibitors is predictive of disease course. BMC Cancer 12:162
Kakizoe M, Yao M, Tateishi U et al (2014) The early response of renal cell carcinoma to tyrosine kinase inhibitors evaluated by FDG PET/CT was not influenced by metastatic organ. BMC Cancer 14:390
Chen JL, Appelbaum DE, Kocherginsky M et al (2013) FDG-PET as a predictive biomarker for therapy with everolimus in metastatic renal cell cancer. Cancer Med 2:545–552
Isaacs JS, Jung YJ, Mole DR et al (2005) HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8:143–153
Linehan WM, Srinivasan R, Schmidt LS (2010) The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol 7:277–285
Lucarelli G, Galleggiante V, Rutigliano M et al (2015) Metabolomic profile of glycolysis and the pentose phosphate pathway identifies the central role of glucose-6-phosphate dehydrogenase in clear cell-renal cell carcinoma. Oncotarget 6:13371–13386
Lyrdal D, Boijsen M, Suurküla M et al (2009) Evaluation of sorafenib treatment in metastatic renal cell carcinoma with 2-fluoro-2-deoxyglucose positron emission tomography and computed tomography. Nucl Med Commun 30:519–524
Kayani I, Avril N, Bomanji J et al (2011) Sequential FDG-PET/CT as a biomarker of response to Sunitinib in metastatic clear cell renal cancer. Clin Cancer Res 17:6021–6028
Haissaguerre M, Saucisse N, Cota D (2014) Influence of mTOR in energy and metabolic homeostasis. Mol Cell Endocrinol 397:67–77
Kumar A, Harris TE, Keller SR et al (2008) Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances basal glycogen synthase activity. Mol Cell Biol 28:61–70
Duvel K, Yecies JL, Menon S et al (2010) Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 39:171–183
Mizuno T, Kamai T, Abe H et al (2015) Clinically significant association between the maximum standardized uptake value on 18F-FDG PET and expression of phosphorylated Akt and S6 kinase for prediction of the biological characteristics of renal cell cancer. BMC Cancer 15:1097
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research (No. 25462494 and 16K11021) from the Ministry of Education, Science, Sports and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved by the Yokohama City University Institutional Review Board. Written informed consent was obtained from all patients.
Rights and permissions
About this article
Cite this article
Ito, H., Kondo, K., Kawahara, T. et al. One-month assessment of renal cell carcinoma treated by everolimus using FDG PET/CT predicts progression-free and overall survival. Cancer Chemother Pharmacol 79, 855–861 (2017). https://doi.org/10.1007/s00280-017-3275-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3275-z