Abstract
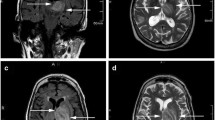
Balamuthia mandrillaris, a free-living ameba, causes rare but frequently fatal granulomatous amebic encephalitis (GAE). Few patients have survived after receiving experimental drug combinations, with or without brain lesion excisions. Some GAE survivors have been treated with a multi-drug regimen including miltefosine, an investigational anti-leishmanial agent with in vitro amebacidal activity. Miltefosine dosing for GAE has been based on leishmaniasis dosing because no data exist in humans concerning its pharmacologic distribution in the central nervous system. We describe results of limited cerebrospinal fluid (CSF) and serum drug level testing performed during clinical management of a child with fatal GAE who was treated with a multiple drug regimen including miltefosine. Brain biopsy specimens, CSF, and sera were tested for B. mandrillaris using multiple techniques, including culture, real-time polymerase chain reaction, immunohistochemical techniques, and serology. CSF and serum miltefosine levels were determined using a liquid chromatography method coupled to tandem mass spectrometry. The CSF miltefosine concentration on hospital admission day 12 was 0.4 μg/mL. The serum miltefosine concentration on day 37, about 80 h post-miltefosine treatment, was 15.3 μg/mL. These are the first results confirming some blood–brain barrier penetration by miltefosine in a human, although with low-level CSF accumulation. Further evaluation of brain parenchyma penetration is required to determine optimal miltefosine dosing for Balamuthia GAE, balanced with the drug’s toxicity profile. Additionally, the Balamuthia isolate was evaluated by real-time polymerase chain reaction (PCR), demonstrating genetic variability in 18S ribosomal RNA (18S rRNA) sequences and possibly signaling the first identification of multiple Balamuthia strains with varying pathogenicities.


Similar content being viewed by others
References
Ahmad AF, Andrews PW, Kilvington S (2011) Development of a nested PCR for environmental detection of the pathogenic free-living amoeba Balamuthia mandrillaris. J Eukaryot Microbiol 58:269–271. doi:10.1111/j.1550-7408.2011.00541.x
Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst P (2003a) Genotyping of Balamuthia mandrillaris based on nuclear 18S and mitochondrial 16S rRNA genes. Am J Trop Med Hyg 68:65–69
Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA (2003b) Identification of Balamuthia mandrillaris by PCR assay using the mitochondrial 16S rRNA gene as a target. J Clin Microbiol 41:453–455
Bravo FG, Alvarez PJ, Gotuzzo E (2011) Balamuthia mandrillaris infection of the skin and central nervous system: an emerging disease of concern to many specialties in medicine. Curr Opin Infect Dis 24:112–117. doi:10.1097/QCO.0b013e3283428d1e
Cary LC, Maul E, Potter C, Wong P, Nelson PT, Given C 2nd, Robertson W Jr (2010) Balamuthia mandrillaris meningoencephalitis: survival of a pediatric patient. Pediatrics 125:e699–e703. doi:10.1542/peds.2009-1797
Centers for Disease Control and Prevention (2010) Balamuthia mandrillaris transmitted through organ transplantation—Mississippi, 2009. MMWR Morb Mortal Wkly Rep 59:1165–1170
Centers for Disease Control and Prevention (2013) Investigational drug available directly from CDC for the treatment of infections with free-living amebae. MMWR Morb Mortal Wkly Rep 62:666
Deetz TR, Sawyer MH, Billman G, Schuster FL, Visvesvara GS (2003) Successful treatment of Balamuthia amoebic encephalitis: presentation of two cases. Clin Infect Dis 37:1304–1312
Dorlo TPC, Hillebrand MJX, Rosing H, Eggelte TA, de Vries PJ, Beijnen JH (2008a) Development and validation of a quantitative assay for the measurement of miltefosine in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 865:55–62. doi:10.1016/j.jchromb.2008.02.005
Dorlo TP, van Thiel PP, Huitema AD, Keizer RJ, de Vries HJ, Beijnen JH, de Vries PJ (2008b) Pharmacokinetics of miltefosine in Old World cutaneous leishmaniasis patients. Antimicrob Agents Chemother 52:2855–2860. doi:10.1128/AAC.00014-08
Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ (2012a) Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother 67:2576–2597. doi:10.1093/jac/dks275
Dorlo TP, Huitema AD, Beijnen JH, de Vries PJ (2012b) Optimal dosing of miltefosine in children and adults with visceral leishmaniasis. Antimicrob Agents Chemother 56:3864–3872. doi:10.1128/AAC.00292-12
Food and Drug Administration (2015) Impavido/Miltefosine—label and approval history. In: Drugs@FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist. Accessed 01 July 2015.
Dunnebacke TH, Schuster FL, Yagi S, Booton GC (2004) Balamuthia mandrillaris from soil samples. Microbiology 150:2837–2842
Eibl H, Unger C (1990) Hexadecylphosphocholine: a new and selective antitumor drug. Cancer Treat Rev 17:233–242
Finnin PJ, Visvesvara GS, Campbell BE, Fry DR, Gasser RB (2007) Multifocal Balamuthia mandrillaris infection in a dog in Australia. Parasitol Res 100:423–426
Foreman O, Sykes J, Ball L, Yang N, De Cock H (2004) Disseminated infection with Balamuthia mandrillaris in a dog. Vet Pathol 41:506–510
Huang ZH, Ferrante A, Carter RF (1999) Serum antibodies to Balamuthia mandrillaris, a free-living amoeba recently demonstrated to cause granulomatous amoebic encephalitis. J Infect Dis 179:1305–1208
Jaruratanasirikul S, Hortiwakul R, Tantisarasart T, Phuenpathom N, Tussanasunthornwong S (1996) Distribution of azithromycin into brain tissue, cerebrospinal fluid, and aqueous humor of the eye. Antimicrob Agents Chemother 40:825–826
Jung S, Schelper RL, Visvesvara GS, Chang HT (2004) Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient: an unusual clinical course and a favorable outcome. Arch Pathol Lab Med 128:466–468
Kiderlen AF, Radam E, Tata PS (2009) Assessment of Balamuthia mandrillaris-specific serum antibody by flow cytometry. Parasitol Res 104:663–670. doi:10.1007/s00436-008-1243-6
Kucerova Z, Sriram R, Wilkins PP, Visvesvara GS (2011) Identification of antigenic targets for immunodetection of Balamuthia mandrillaris. Clin Vaccine Immunol 18:1297–1301. doi:10.1128/CVI.05082-11
Marschner M, Kötting J, Eibl H, Unger C (1992) Distribution of hexadecylphosphocholine and octadecyl-methyl-glycero-3-phosphocholine in rat tissues during steady-state treatment. Cancer Chemother Pharmacol 31:18–22
Martínez DY, Seas C, Bravo C, Legua P, Ramos C, Cabello AM, Gotuzzo E (2010) Successful treatment of Balamuthia mandrillaris amoebic infection with extensive neurological and cutaneous involvement. Clin Infect Dis 51:e7–e11. doi:10.1086/653609
Nau R, Sörgel F, Eiffert H (2010) Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev 23:858–883. doi:10.1128/CMR.00007-10
Niyyati M, Lorenzo-Morales J, Rezaeian M, Martin-Navarro CM, Haghi AM, Maciver SK, Valladares B (2009) Isolation of Balamuthia mandrillaris from urban dust, free of known infectious involvement. Parasitol Res 106:279–281. doi:10.1007/s00436-009-1592-9
Petersen CA, Greenlee MHW (2011) Neurologic manifestations of Leishmania spp. infection. J Neuroparasitology 2: doi: 10.4303/jnp/N110401
Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ (2006) Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol 44:3589–3595
Qvarnstrom Y, Nerad TA, Visvesvara GS (2013) Characterization of a new pathogenic Acanthamoeba species, A. byersi n. sp., isolated from a human with fatal amoebic encephalitis. J Eukaryot Microbiol 60:626–633. doi:10.1111/jeu.12069
Reed RP, Cooke-Yarborough CM, Jaquiery AL, Grimwood K, Kemp AS, Su JC, Forsyth JRL (1997) Fatal granulomatous amoebic encephalitis caused by Balamuthia mandrillaris. Med J Aust 167:82–84
Schuster FL, Visvesvara GS (1996) Axenic growth and drug sensitivity studies of Balamuthia mandrillaris, an agent of amebic meningoencephalitis in humans and other animals. J Clin Microbiol 34:385–388
Schuster FL, Dunnebacke TH, Booton GC, Yagi S, Kohlmeier CK, Glaser C, Vugia D, Bakardjiev A, Azimi P, Maddux-Gonzalez M, Visvesvara GS (2003) Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. J Clin Microbiol 41:3175–3180
Schuster FL, Glaser C, Honarmand S, Maguire JH, Visvesvara GS (2004) Balamuthia amebic encephalitis risk, Hispanic Americans. Emerg Infect Dis 10:1510–1512
Schuster FL, Guglielmo BJ, Visvesvara GS (2006a) In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amoebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol 53:121–126
Schuster FL, Honarmand S, Visvesvara GS, Glaser CA (2006b) Detection of antibodies against free-living amoebae Balamuthia mandrillaris and Acanthamoeba species in a population of patients with encephalitis. Clin Infect Dis 42:1260–1265
Schuster FL, Yagi S, Gavali S, Michelson D, Raghavan R, Blomquist I, Glastonbury C, AW B e, Scharnhorst D, Reed SL, Kuriyama S, Visvesvara GS, Glaser CA (2009) Under the radar: Balamuthia amebic encephalitis. Clin Infect Dis 7:879–887. doi:10.1086/597260
U.S. Food and Drug Administration (2014) Impavido (Miltefosine). Drugs@FDA—FDA approved drug products. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. Accessed 27 March 2015
Visvesvara GS, Martinez AJ, Schuster FL, Leitch GJ, Wallace SV, Sawyer TK, Anderson M (1990) Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol 28:2750–2756
Visvesvara GS, Schuster FL, Martinez AJ (1993) Balamuthia mandrillaris, N. G., N. Sp., agent of amebic meningoencephalitis in humans and animals. J Eukaryot Microbiol 40:504–514
Visvesvara GS, Roy S, Maguire JH (2011) Pathogenic and opportunistic free-living amebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia pedata. In: Guerrant RL, Walker DH, Weller PF (eds) Tropical infectious diseases—principles, pathogens, & practice, 3rd edn. Elsevier, Churchill Livingstone, Philadelphia, pp 707–713
Wadhone P, Maiti M, Agarwal R, Kamat V, Martin S, Saha B (2009) Miltefosine promotes IFN-gamma-dominated anti-leishmanial immune response. J Immunol 182:7146–7154. doi:10.4049/jimmunol.0803859
World Health Organization (2011) Miltefosine (Inclusion)—adults and children. 18th expert committee on the selection and use of essential medicines. World Health Organization. http://www.who.int/selection_medicines/committees/expert/18/applications/miltefosine/en/. Accessed 27 March 2015
Conflict of interest
The authors declare that they have no conflicts of interest.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roy, S.L., Atkins, J.T., Gennuso, R. et al. Assessment of blood–brain barrier penetration of miltefosine used to treat a fatal case of granulomatous amebic encephalitis possibly caused by an unusual Balamuthia mandrillaris strain. Parasitol Res 114, 4431–4439 (2015). https://doi.org/10.1007/s00436-015-4684-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4684-8