Abstract
Introduction
Neoadjuvant chemotherapy in breast cancer is used to downstage locally advanced and inoperable tumors. Expanded benefits of neoadjuvant chemotherapy include downstaging of tumors to allow breast-conserving surgery (BCS) and assessment of in vivo tumor response. We sought to identify patterns and predictors of neoadjuvant chemotherapy use to determine if this has translated into population-level clinical practice.
Methods
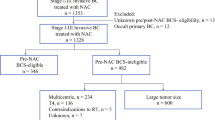
All patients undergoing surgery for invasive breast cancer between January 2012 and June 2014 were identified from our provincial synoptic operating room database. Data regarding patient demographics, hospital, operating surgeon, preoperative tumor characteristics, neoadjuvant treatment, and type of surgery performed were collected. Descriptive statistics and multivariable analysis were used to identify predictors of neoadjuvant chemotherapy.
Results
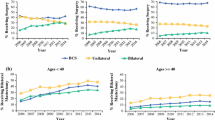
A total of 4186 patients were identified, 363 (8.53 %) of whom underwent neoadjuvant chemotherapy. A significant increase was seen in the use of neoadjuvant chemotherapy over time. In multivariable analysis, neoadjuvant chemotherapy was associated with prechemotherapy tumor size, multicentricity, lymph node positivity, and decreasing patient age. In addition, there was significant variability in neoadjuvant chemotherapy use between operating surgeons. Of those patients who underwent neoadjuvant chemotherapy, 68.9 % were not pretreatment candidates for BCS. At the time of definitive surgery, 72.1 % had mastectomy, with 18.7 % opting for contralateral prophylactic mastectomy. As reported, this was due to the tumor being advanced/too large (50.4 %), patient preference (12.6 %), multicentricity (8.8 %) and margins, genetics, and previous radiotherapy (4 %).
Conclusions
A significant increase in the use of neoadjuvant chemotherapy over time was identified, and treatment with mastectomy as definitive surgical management remained high. There was significant variability in neoadjuvant chemotherapy use by the operating surgeons, in addition to factors generally associated with more locally advanced/aggressive tumors.

Similar content being viewed by others
References
Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102.
Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: national surgical adjuvant breast and bowel project protocol B-27. J Clin Oncol. 2006;24:2019–27.
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85.
Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188–194.
Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94:1189–200.
Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–69.
Guarneri V, Broglio K, Kau SW, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037–44.
Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23:9304–11.
Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–72.
Alberta Provincial Breast Tumor Board Team. Neo-adjuvant (pre-operative) therapy for breast cancer: general considerations. www.albertahealthservices.ca/hp/if-hp-cancer-guide-br015.pdf (2014). Accessed Dec 2014.
Madarnas Y, Mates M, Agbassi C. The role of taxanes in neoadjuvant chemotherapy for women with non-metastatic breast cancer. https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=13888 (2011). Accessed 5 Oct 2011.
Madarnas Y, Tey R. The role of trastuzumab in adjuvant and neoadjuvant therapy in women with HER2/neu-overexpressing breast cancer. https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=13892 (2011). Accessed 15 Sept 2011.
Chin-Lenn L, Mack LA, Temple W, et al. Predictors of treatment with mastectomy, use of sentinel lymph node biopsy and upstaging to invasive cancer in patients diagnosed with breast ductal carcinoma in situ (DCIS) on core biopsy. Ann Surg Oncol. 2014;21:66–73.
Chang HR. Trastuzumab-based neoadjuvant therapy in patients with HER2-positive breast cancer. Cancer. 2010;116:2856–67.
Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–40.
Robidoux A, Tang G, Rastogi P, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1183–92.
Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85.
Kling KM, Ostrzega N, Schmit P. Breast conservation after induction chemotherapy for locally advanced breast cancer. Am Surg. 1997;63:861–64.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–18.
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (alliance) clinical trial. JAMA. 2013;310:1455–61.
Alliance for Clinical Trials in Oncology. Comparison of axillary lymph node dissection with axillary radiation for patients with node-positive breast cancer treated with chemotherapy [ClinicalTrials.gov identifier NCT01901094]. US National Institutes of Health, ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01901094 (2014). Accessed 6 Nov 2014.
National Surgical Adjuvant Breast and Bowel Proejct (NSABP) Foundation, Inc. Standard or comprehensive radiation therapy in treating patients with early-stage breast cancer previously treated with chemotherapy and surgery [ClinicalTrials.gov identifier 01872975]. US National Institutes of Health, ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01872975 (2015). Accessed 6 April 2015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graham, P.J., Brar, M.S., Foster, T. et al. Neoadjuvant Chemotherapy for Breast Cancer, Is Practice Changing? A Population-Based Review of Current Surgical Trends. Ann Surg Oncol 22, 3376–3382 (2015). https://doi.org/10.1245/s10434-015-4714-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4714-x