Key Points
-
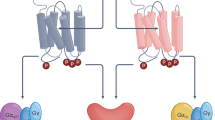
The five muscarinic acetylcholine receptors (mAChRs) are prototypical class A G protein-coupled receptors (GPCRs).
-
mAChRs regulate many fundamental functions of the central and peripheral nervous system.
-
Recent studies with novel mAChR mouse models have provided detailed insights into the physiological roles of the different mAChR subtypes (M1 to M5).
-
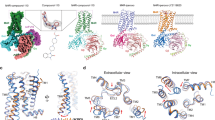
High-resolution structural information is now available for the M2 and M3 receptors, providing a structural basis for mAChR activation and the binding of different types of muscarinic ligands, including allosteric modulators.
-
These new findings should facilitate the development of novel drugs targeting muscarinic receptors for the treatment of many severe pathophysiological conditions.
Abstract
The muscarinic acetylcholine receptors are a subfamily of G protein-coupled receptors that regulate numerous fundamental functions of the central and peripheral nervous system. The past few years have witnessed unprecedented new insights into muscarinic receptor physiology, pharmacology and structure. These advances include the first structural views of muscarinic receptors in both inactive and active conformations, as well as a better understanding of the molecular underpinnings of muscarinic receptor regulation by allosteric modulators. These recent findings should facilitate the development of new muscarinic receptor subtype-selective ligands that could prove to be useful for the treatment of many severe pathophysiological conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fredriksson, R., Lagerstrom, M. C., Lundin, L. G. & Schioth, H. B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63, 1256–1272 (2003).
Hulme, E. C., Birdsall, N. J. & Buckley, N. J. Muscarinic receptor subtypes. Annu. Rev. Pharmacol. Toxicol. 30, 633–673 (1990).
Wess, J., Eglen, R. M. & Gautam, D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nature Rev. Drug Discov. 6, 721–733 (2007).
Wess, J. Novel muscarinic receptor mutant mouse models. Handb. Exp. Pharmacol. 208, 95–117 (2012).
Conn, P. J., Jones, C. K. & Lindsley, C. W. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol. Sci. 30, 148–155 (2009).
Wootten, D., Christopoulos, A. & Sexton, P. M. Emerging paradigms in GPCR allostery: implications for drug discovery. Nature Rev. Drug Discov. 12, 630–644 (2013).
Lane, J. R., Sexton, P. M. & Christopoulos, A. Bridging the gap: bitopic ligands of G-protein-coupled receptors. Trends Pharmacol. Sci. 34, 59–66 (2013).
Bock, A. & Mohr, K. Dualsteric GPCR targeting and functional selectivity: the paradigmatic M2 muscarinic acetylcholine receptor. Drug Discov. Today Technol. 10, e245–e252 (2013).
De Amici, M., Dallanoce, C., Holzgrabe, U., Tränkle, C. & Mohr, K. Allosteric ligands for G protein-coupled receptors: a novel strategy with attractive therapeutic opportunities. Med. Res. Rev. 30, 463–549 (2010).
Kruse, A. C. et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 482, 552–556 (2012). This study reports the first high-resolution structure of the M 3 receptor in complex with tiotropium, a clinically used muscarinic antagonist and inverse agonist.
Haga, K. et al. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature 482, 547–551 (2012). In this study, the authors present the first high-resolution structure of the M 2 receptor in complex with an orthosteric muscarinic antagonist and inverse agonist — QNB.
Kruse, A. C. et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504, 101–106 (2013). This study provides the first high-resolution structural information of an agonist-activated mAChR (the M 2 subtype) and reveals how a PAM interacts with the M 2 receptor.
Dror, R. O. et al. Structural basis for modulation of a G-protein-coupled receptor by allosteric drugs. Nature 503, 295–299 (2013). This study represents a computational biology breakthrough in delineating the molecular mechanisms governing the allosteric modulation of the M 2 receptor.
Ballard, C. et al. Alzheimer's disease. Lancet 377, 1019–1031 (2011).
Langmead, C. J., Watson, J. & Reavill, C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 117, 232–243 (2008).
Davis, A. A., Fritz, J. J., Wess, J., Lah, J. J. & Levey, A. I. Deletion of M1 muscarinic acetylcholine receptors increases amyloid pathology in vitro and in vivo. J. Neurosci. 30, 4190–4196 (2010).
Medeiros, R. et al. Loss of muscarinic M1 receptor exacerbates Alzheimer's disease-like pathology and cognitive decline. Am. J. Pathol. 179, 980–991 (2011).
Melancon, B. J., Tarr, J. C., Panarese, J. D., Wood, M. R. & Lindsley, C. W. Allosteric modulation of the M1 muscarinic acetylcholine receptor: improving cognition and a potential treatment for schizophrenia and Alzheimer's disease. Drug Discov. Today 18, 1185–1199 (2013).
Davie, B. J., Christopoulos, A. & Scammells, P. J. Development of M1 mAChR allosteric and bitopic ligands: prospective therapeutics for the treatment of cognitive deficits. ACS Chem. Neurosci. 4, 1026–1048 (2013).
van Os, J. & Kapur, S. Schizophrenia. Lancet 374, 635–645 (2009).
McKinzie, D. L. & Bymaster, F. P. Muscarinic mechanisms in psychotic disorders. Handb. Exp. Pharmacol. 213, 233–265 (2012).
Bodick, N. C. et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch. Neurol. 54, 465–473 (1997).
Shekhar, A. et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry 165, 1033–1039 (2008).
Jeon, J. et al. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J. Neurosci. 30, 2396–2405 (2010).
Dencker, D. et al. Involvement of a subpopulation of neuronal M4 muscarinic acetylcholine receptors in the antipsychotic-like effects of the M1/M4 preferring muscarinic receptor agonist xanomeline. J. Neurosci. 31, 5905–5908 (2011).
Foster, D. J., Jones, C. K. & Conn, P. J. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov. Med. 14, 413–420 (2012).
Jones, C. K., Byun, N. & Bubser, M. Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology 37, 16–42 (2012).
Dencker, D. et al. Muscarinic acetylcholine receptor subtypes as potential drug targets for the treatment of schizophrenia, drug abuse and Parkinson's disease. ACS Chem. Neurosci. 3, 80–89 (2012).
Sofuoglu, M. & Mooney, M. Cholinergic functioning in stimulant addiction: implications for medications development. CNS Drugs 23, 939–952 (2009).
Thomsen, M. et al. Attenuation of cocaine's reinforcing and discriminative stimulus effects via muscarinic M1 acetylcholine receptor stimulation. J. Pharmacol. Exp. Ther. 332, 959–969 (2010).
Thomsen, M. et al. Contribution of both M1 and M4 receptors to muscarinic agonist-mediated attenuation of the cocaine discriminative stimulus in mice. Psychopharmacol. 220, 673–685 (2012).
Schmidt, L. S. et al. Increased cocaine self-administration in M4 muscarinic acetylcholine receptor knockout mice. Psychopharmacol. 216, 367–378 (2011).
Lam, D. W. & LeRoith, D. The worldwide diabetes epidemic. Curr. Opin. Endocrinol. Diabetes Obes. 19, 93–96 (2012).
Gautam, D. et al. A critical role for β cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell. Metab. 3, 449–461 (2006).
Gautam, D. et al. Beneficial metabolic effects caused by persistent activation of β-cell M3 muscarinic acetylcholine receptors in transgenic mice. Endocrinology 151, 5185–5194 (2010).
Guettier, J. M. et al. A chemical-genetic approach to study G protein regulation of β cell function in vivo. Proc. Natl Acad. Sci. USA 106, 19197–19202 (2009).
Jain, S. et al. Chronic activation of a designer Gq-coupled receptor improves β cell function. J. Clin. Invest. 123, 1750–1762 (2013). This study shows that chronic, exogenous ligand-induced activation of an M 3 receptor-derived designer receptor expressed by pancreatic β -cells prevents diabetes in different mouse models.
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Sassmann, A. et al. The Gq/G11-mediated signaling pathway is critical for autocrine potentiation of insulin secretion in mice. J. Clin. Invest. 120, 2184–2193 (2010).
Kong, K. C. et al. M3-muscarinic receptor promotes insulin release via receptor phosphorylation/arrestin-dependent activation of protein kinase D1. Proc. Natl Acad. Sci. USA 107, 21181–21186 (2010). This analysis of phosphorylation-deficient M 3 receptor knock-in mice strongly suggests that arrestin-dependent signalling pathways contribute to M 3 receptor-stimulated insulin release.
Nakajima, K. & Wess, J. Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol. Pharmacol. 82, 575–582 (2012).
Shah, N., Khurana, S., Cheng, K. & Raufman, J. P. Muscarinic receptors and ligands in cancer. Am. J. Physiol. Cell Physiol. 296, C221–C232 (2009).
Spindel, E. R. Muscarinic receptor agonists and antagonists: effects on cancer. Handb. Exp. Pharmacol. 208, 451–468 (2012).
Raufman, J. P. et al. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res. 68, 3573–3578 (2008).
Raufman, J. P. et al. Muscarinic receptor subtype-3 gene ablation and scopolamine butylbromide treatment attenuate small intestinal neoplasia in Apcmin/+ mice. Carcinogenesis 32, 1396–1402 (2011).
Magnon, C. et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361 (2013). This study reports that M 1 receptor deficiency inhibits mAChR-mediated prostate cancer invasion and metastasis in two mouse models of prostate cancer.
Christopoulos, A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nature Rev. Drug Discov. 1, 198–210 (2002).
May, L. T., Leach, K., Sexton, P. M. & Christopoulos, A. Allosteric modulation of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 47, 1–51 (2007).
Christopoulos, A., Lanzafame, A. & Mitchelson, F. Allosteric interactions at muscarinic cholinoceptors. Clin. Exp. Pharmacol. Physiol. 25, 185–194 (1998).
Birdsall, N. J. & Lazareno, S. Allosterism at muscarinic receptors: ligands and mechanisms. Mini Rev. Med. Chem. 5, 523–543 (2005).
Conn, P. J., Christopoulos, A. & Lindsley, C. W. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nature Rev. Drug Discov. 8, 41–54 (2009).
Keov, P., Sexton, P. M. & Christopoulos, A. Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology 60, 24–35 (2011).
Keov, P. et al. Reverse engineering of the selective agonist TBPB unveils both orthosteric and allosteric modes of action at the M1 muscarinic acetylcholine receptor. Mol. Pharmacol. 84, 425–437 (2013).
Kenakin, T. & Christopoulos, A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nature Rev. Drug Discov. 12, 205–216 (2013).
Ma, L. et al. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc. Natl Acad. Sci. USA 106, 15950–15955 (2009).
Shirey, J. K. et al. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J. Neurosci. 29, 14271–14286 (2009).
Canals, M. et al. A Monod–Wyman–Changeux mechanism can explain G protein-coupled receptor (GPCR) allosteric modulation. J. Biol. Chem. 287, 650–659 (2012). This study presents a chemical biology framework with which to study and classify the simplest allosteric ligand behaviours.
Lazareno, S. & Birdsall, N. J. Detection, quantitation, and verification of allosteric interactions of agents with labeled and unlabeled ligands at G protein-coupled receptors: interactions of strychnine and acetylcholine at muscarinic receptors. Mol. Pharmacol. 48, 362–378 (1995).
Kenakin, T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nature Rev. Drug Discov. 4, 919–927 (2005).
Valant, C., Felder, C. C., Sexton, P. M. & Christopoulos, A. Probe dependence in the allosteric modulation of a G protein-coupled receptor: implications for detection and validation of allosteric ligand effects. Mol. Pharmacol. 81, 41–52 (2012). This study highlights the importance of probe dependence in the study of the effects of allosteric modulators.
Rajagopal, S., Rajagopal, K. & Lefkowitz, R. J. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nature Rev. Drug Discov. 9, 373–386 (2010).
Stallaert, W., Christopoulos, A. & Bouvier, M. Ligand functional selectivity and quantitative pharmacology at G protein-coupled receptors. Expert Opin. Drug Discov. 6, 811–825 (2011).
Marlo, J. E. et al. Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol. Pharmacol. 75, 577–588 (2009).
Lazareno, S., Dolezal, V., Popham, A. & Birdsall, N. J. Thiochrome enhances acetylcholine affinity at muscarinic M4 receptors: receptor subtype selectivity via cooperativity rather than affinity. Mol. Pharmacol. 65, 257–266 (2004).
Chan, W. Y. et al. Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc. Natl Acad. Sci. USA 105, 10978–10983 (2008).
Suratman, S. et al. Impact of species variability and 'probe-dependence' on the detection and in vivo validation of allosteric modulation at the M4 muscarinic acetylcholine receptor. Br. J. Pharmacol. 162, 1659–1670 (2011).
Valant, C., Sexton, P. M. & Christopoulos, A. Orthosteric/allosteric bitopic ligands: going hybrid at GPCRs. Mol. Interv. 9, 125–135 (2009).
Mohr, K. et al. Rational design of dualsteric GPCR ligands: quests and promise. Br. J. Pharmacol. 159, 997–1008 (2010).
Melancon, B. J. et al. Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J. Med. Chem. 55, 1445–1464 (2012).
Valant, C., Robert Lane, J., Sexton, P. M. & Christopoulos, A. The best of both worlds? Bitopic orthosteric/allosteric ligands of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 52, 153–178 (2012).
Disingrini, T. et al. Design, synthesis, and action of oxotremorine-related hybrid-type allosteric modulators of muscarinic acetylcholine receptors. J. Med. Chem. 49, 366–372 (2006).
Antony, J. et al. Dualsteric GPCR targeting: a novel route to binding and signaling pathway selectivity. FASEB J. 23, 442–450 (2009).
Kebig, A., Kostenis, E., Mohr, K. & Mohr-Andra, M. An optical dynamic mass redistribution assay reveals biased signaling of dualsteric GPCR activators. J. Recept. Signal Transduct. Res. 29, 140–145 (2009).
Bock, A. et al. The allosteric vestibule of a seven transmembrane helical receptor controls G-protein coupling. Nature Commun. 3, 1044 (2012).
Steinfeld, T., Mammen, M., Smith, J. A., Wilson, R. D. & Jasper, J. R. A novel multivalent ligand that bridges the allosteric and orthosteric binding sites of the M2 muscarinic receptor. Mol. Pharmacol. 72, 291–302 (2007).
Valant, C. et al. A novel mechanism of G protein-coupled receptor functional selectivity. Muscarinic partial agonist McN-A-343 as a bitopic orthosteric/allosteric ligand. J. Biol. Chem. 283, 29312–29321 (2008). This is the first study to show that functionally selective ligands may mediate their behaviour via a bitopic mechanism.
Spalding, T. A. et al. Discovery of an ectopic activation site on the M1 muscarinic receptor. Mol. Pharmacol. 61, 1297–1302 (2002).
Langmead, C. J. et al. Probing the molecular mechanism of interaction between 4-n-butyl-1-[4-(2-methylphenyl)-4-oxo-1-butyl]-piperidine (AC-42) and the muscarinic M1 receptor: direct pharmacological evidence that AC-42 is an allosteric agonist. Mol. Pharmacol. 69, 236–246 (2006).
Jones, C. K. et al. Novel selective allosteric activator of the M1 muscarinic acetylcholine receptor regulates amyloid processing and produces antipsychotic-like activity in rats. J. Neurosci. 28, 10422–10433 (2008).
Gregory, K. J., Hall, N. E., Tobin, A. B., Sexton, P. M. & Christopoulos, A. Identification of orthosteric and allosteric site mutations in M2 muscarinic acetylcholine receptors that contribute to ligand-selective signaling bias. J. Biol. Chem. 285, 7459–7474 (2010).
Avlani, V. A. et al. Orthosteric and allosteric modes of interaction of novel selective agonists of the M1 muscarinic acetylcholine receptor. Mol. Pharmacol. 78, 94–104 (2010).
Monod, J., Wyman, J. & Changeux, J. P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12, 88–118 (1965).
Changeux, J. P. Allosteric receptors: from electric organ to cognition. Annu. Rev. Pharmacol. Toxicol. 50, 1–38 (2010).
Canals, M., Sexton, P. M. & Christopoulos, A. Allostery in GPCRs: 'MWC' revisited. Trends Biochem. Sci. 36, 663–672 (2011).
Ehlert, F. J. & Griffin, M. T. Two-state models and the analysis of the allosteric effect of gallamine at the M2 muscarinic receptor. J. Pharmacol. Exp. Ther. 325, 1039–1060 (2008).
Abdul-Ridha, A., Lane, J. R., Sexton, P. M., Canals, M. & Christopoulos, A. Allosteric modulation of a chemogenetically modified G protein-coupled receptor. Mol. Pharmacol. 83, 521–530 (2013).
Lazareno, S., Popham, A. & Birdsall, N. J. Allosteric interactions of staurosporine and other indolocarbazoles with N-[methyl-3H] scopolamine and acetylcholine at muscarinic receptor subtypes: identification of a second allosteric site. Mol. Pharmacol. 58, 194–207 (2000).
Lazareno, S., Popham, A. & Birdsall, N. J. Analogs of WIN 62,577 define a second allosteric site on muscarinic receptors. Mol. Pharmacol. 62, 1492–1505 (2002).
Espinoza-Fonseca, L. M. & Trujillo-Ferrara, J. G. The existence of a second allosteric site on the M1 muscarinic acetylcholine receptor and its implications for drug design. Bioorg. Med. Chem. Lett. 16, 1217–1220 (2006).
Redka, D. S., Pisterzi, L. F. & Wells, J. W. Binding of orthosteric ligands to the allosteric site of the M2 muscarinic cholinergic receptor. Mol. Pharmacol. 74, 834–843 (2008).
Shivnaraine, R. V., Huang, X. P., Seidenberg, M., Ellis, J. & Wells, J. W. Heterotropic cooperativity within and between protomers of an oligomeric M2 muscarinic receptor. Biochemistry 51, 4518–4540 (2012).
Rosenbaum, D. M. et al. GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science 318, 1266–1273 (2007).
Chae, P. S. et al. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nature Methods 7, 1003–1008 (2010).
Landau, E. M. & Rosenbusch, J. P. Lipidic cubic phases: a novel concept for the crystallization of membrane proteins. Proc. Natl Acad. Sci. USA 93, 14532–14535 (1996).
Caffrey, M. & Cherezov, V. Crystallizing membrane proteins using lipidic mesophases. Nature Protoc. 4, 706–731 (2009).
Smith, J. L., Fischetti, R. F. & Yamamoto, M. Micro-crystallography comes of age. Curr. Opin. Struct. Biol. 22, 602–612 (2012).
Shimamura, T. et al. Structure of the human histamine H1 receptor complex with doxepin. Nature 475, 65–70 (2011).
Chien, E. Y. et al. Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 330, 1091–1095 (2010).
Warne, T. et al. Structure of a β1-adrenergic G-protein-coupled receptor. Nature 454, 486–491 (2008).
Rasmussen, S. G. et al. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature 450, 383–387 (2007).
Cherezov, V. et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 (2007).
Wacker, D. et al. Structural features for functional selectivity at serotonin receptors. Science 340, 615–619 (2013).
Wang, C. et al. Structural basis for molecular recognition at serotonin receptors. Science 340, 610–614 (2013).
Ballesteros, J. & Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25, 366–428 (1995).
Kruse, A. C. et al. Muscarinic receptors as model targets and antitargets for structure-based ligand discovery. Mol. Pharmacol. 84, 528–540 (2013).
Tautermann, C. S. et al. Molecular basis for the long duration of action and kinetic selectivity of tiotropium for the muscarinic M3 receptor. J. Med. Chem. 56, 8746–8756 (2013).
Gregory, K. J., Sexton, P. M. & Christopoulos, A. Allosteric modulation of muscarinic acetylcholine receptors. Curr. Neuropharmacol. 5, 157–167 (2007).
Nygaard, R. et al. The dynamic process of β2-adrenergic receptor activation. Cell 152, 532–542 (2013).
Scheerer, P. et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature 455, 497–502 (2008).
Choe, H. W. et al. Crystal structure of metarhodopsin II. Nature 471, 651–655 (2011).
Standfuss, J. et al. The structural basis of agonist-induced activation in constitutively active rhodopsin. Nature 471, 656–660 (2011).
Rasmussen, S. G. et al. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469, 175–180 (2011).
Rasmussen, S. G. et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555 (2011). This crystal structure represents the first high-resolution view of the active-state ternary complex composed of an agonist-occupied GPCR (β 2 -AR) and a G protein (nucleotide-free G s heterotrimer).
Lebon, G. et al. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature 474, 521–525 (2011).
Xu, F. et al. Structure of an agonist-bound human A2A adenosine receptor. Science 332, 322–327 (2011).
Kamsler, A., McHugh, J., Gerber, D., Huang, S. Y. & Tonegawa, S. Presynaptic M1 muscarinic receptors are necessary for mGluR long-term depression in the hippocampus. Proc. Natl Acad. Sci. USA 107, 1618–1623 (2010).
Gautam, D. et al. Neuronal M3 muscarinic acetylcholine receptors are essential for somatotroph proliferation and normal somatic growth. Proc. Natl Acad. Sci. USA 106, 6398–6403 (2009).
Shi, Y. et al. Signaling through the M3 muscarinic receptor favors bone mass accrual by decreasing sympathetic activity. Cell. Metab. 11, 231–238 (2010).
Li, J. H. et al. Hepatic muscarinic acetylcholine receptors are not critically involved in maintaining glucose homeostasis in mice. Diabetes 58, 2776–2787 (2009).
Arteaga-Solis, E. et al. Inhibition of leptin regulation of parasympathetic signaling as a cause of extreme body weight-associated asthma. Cell. Metab. 17, 35–48 (2013). This study reports that leptin signalling in the brain promotes bronchodilation by inhibiting parasympathetic signalling through airway smooth muscle M 3 receptors.
Poulin, B. et al. The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc. Natl Acad. Sci. USA 107, 9440–9445 (2010).
Bendor, J. et al. AGAP1/AP-3-dependent endocytic recycling of M5 muscarinic receptors promotes dopamine release. EMBO J. 29, 2813–2826 (2010).
Acknowledgements
We apologize to all investigators whose important contributions could not be acknowledged owing to space limitations. The work of A.C.K. and B.K.K. was supported by a US National Science Foundation Graduate Research Fellowship (A.C.K.) and by the National Science Foundation grant CHE-1223785 and US National Institutes of Health (NIH) grant U19GM106990 (B.K.K.). A.C. and P.M.S. received funds from Program Grant No. APP1055134 of the National Health and Medical Research Council (NHMRC) of Australia. A.C. and P.M.S. are NHMRC Principal Research Fellows. The research of D.G. and J.W. was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the NIH. We thank all our co-workers and collaborators for their invaluable contributions to the work summarized in this Review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kruse, A., Kobilka, B., Gautam, D. et al. Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat Rev Drug Discov 13, 549–560 (2014). https://doi.org/10.1038/nrd4295
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd4295
This article is cited by
-
Molecular mechanism of muscarinic acetylcholine receptor M3 interaction with Gq
Communications Biology (2024)
-
Surface-Tailored Nanoplatform for the Diagnosis and Management of Stroke: Current Strategies and Future Outlook
Molecular Neurobiology (2024)
-
Structural basis of efficacy-driven ligand selectivity at GPCRs
Nature Chemical Biology (2023)
-
Determination of Region-Specific Roles of the M3 Muscarinic Acetylcholine Receptor in Gastrointestinal Motility
Digestive Diseases and Sciences (2023)
-
Balanced modulation of neuromuscular synaptic transmission via M1 and M2 muscarinic receptors during inhibition of cholinesterases
Scientific Reports (2022)