Abstract
This prospective, double-blinded, randomized controlled study aimed to investigate the efficacy and safety of oral tadalafil in patients receiving background ambrisentan therapy. Current treatments for pulmonary arterial hypertension (PAH) remain insufficient, resulting in high mortality rates. The addition of oral tadalafil, a phosphodiesterase-5 inhibitor, to background ambrisentan may provide a safe and effective therapeutic strategy. A total of 124 patients who had been treated with ambrisentan for at least 4 months and had a stable 6-min walking distance (6MWD) and World Health Organization (WHO) functional class (FC) for at least 1 month were randomized to either the oral tadalafil or placebo group. Treatment differences in 6MWD, changes in FC, clinical worsening (CW) and adverse events were analyzed after 16 weeks of treatment. At week 16, the tadalafil group showed a significantly improved exercise capacity as assessed by the 6MWD (P<0.05). In addition, 5 (8.3%) patients receiving tadalafil add-on therapy had CW vs. 15 (23.4%) with placebo (P<0.05). No significant differences were found in adverse events or changes in hemodynamic parameters between the placebo and tadalafil groups. Tadalafil 40 mg was well-tolerated as add-on therapy to background ambrisentan. However, the data from this study are insufficient to prove the additional therapeutic benefits of tadalafil add-on therapy.
Similar content being viewed by others
Introduction
Pulmonary arterial hypertension (PAH) remains a devastating chronic and progressive disease, characterized by elevations in the mean pulmonary arterial pressure and pulmonary vascular resistance (PVR).1 The resulting increases in pulmonary arterial pressure and PVR frequently lead to extensive impairment of right ventricular function, eventually resulting in right heart failure and death.2 PAH carries a grave prognosis, with a median survival of 2.8 years for untreated patients.3 In the last decade, novel treatments have been developed, studied and approved for use in patients with PAH, including prostacyclins (epoprostenol, treprostinil and iloprost), endothelin (ET) receptor antagonists (bosentan and ambrisentan) and phosphodiesterase (PDE) inhibitors (sildenafil and tadalafil). With these treatments, PAH patients have shown improvement in symptoms and exercise tolerance. However, none of these treatments provide a cure for PAH or have demonstrated optimal long-term therapeutic outcomes.
ET-1 is a potent vasoconstrictor that has a key role in the pathophysiology of PAH, binding to two distinct receptor isoforms in the pulmonary vascular smooth muscle cells: ET-A and ET-B receptors.4 ET-1 receptor antagonists approved for the treatment of PAH include the nonselective ETA/ETB receptor antagonist bosentan and the selective ETA receptor antagonist ambrisentan. Both agents block ETA receptor-dependent vascular smooth muscle contraction, whereas ambrisentan does not reduce ET-1-mediated nitric oxide production or ETB receptor-mediated ET-1 clearance.5, 6 In addition, ambrisentan has fewer drug interactions and is related to only mild adverse effects on the liver.7
The vascular endothelium and airway epithelium in the lung produce nitric oxide, which can cause vasodilation by activating guanylate cyclase and subsequent cyclic guanosine monophosphate-dependent protein kinase G. It has been reported that patients with PAH have increased expression of PDE-5, leading to reduced cyclic guanosine monophosphate. Therefore, PDE-5 inhibitors provide another treatment strategy for PAH. PDE-5 inhibitors preserve cyclic guanosine monophosphate in the nitric oxide-cyclic guanosine monophosphate-protein kinase G signaling pathway, resulting in vasodilation. Both sildenafil and tadalafil have been approved for the clinical treatment of PAH. However, the possible once-daily dosing scheme because of its longer half-life of approximately 17–18 h makes tadalafil a more favorable option. On the basis of the multifactorial pathophysiology that involves more than one signaling pathway, using a combination of agents targeting different pathogenic mechanisms may provide favorable clinical benefits.8
To date, a few trials of combination therapy have been completed with mixed results.8, 9, 10, 11, 12, 13, 14, 15, 16, 17 A recent clinical study in healthy volunteers showed that the combination of tadalafil and ambrisentan was safe and well tolerated. In addition, no clinical pharmacokinetic interactions or safety issues were reported.18 In this study, we assessed the efficacy and safety of tadalafil or placebo in PAH patients receiving ambrisentan therapy.
Methods
Selection of patients
Between September 2011 and March 2013, 124 patients aged 18–70 years with symptomatic PAH receiving ambrisentan for 4 months or more were enrolled. All patients’ diagnoses were confirmed as either idiopathic/familial PAH or PAH related to anorexigen use, connective tissue disease or repaired congenital heart disease.
Study inclusion requirements included a resting mean pulmonary arterial pressure (mPAP) ⩾25 mm Hg, pulmonary wedge pressure ⩽15 mm Hg and PVR ⩾3 Wood units. All enrolled patients had a 6-min walk distance (6MWD) between 150 and 400 m (6MWD stable for at least 1 month). Patients with thromboembolic disease, untreated obstructive sleep apnea, portal hypertension, chronic liver disease, renal insufficiency, left-sided or unrepaired congenital heart disease, or substantial obstructive (forced expiratory volume in one second/forced vital capacity <50% predicted) or restrictive (total lung capacity <60% predicted) lung disease were excluded. Enrolled patients were also required to have a stable World Health Organization (WHO) functional class (FC) for at least 1 month before study inclusion. Patients were allowed to use concurrent anticoagulants, vasodilators, diuretics, cardiac glycosides or supplemental oxygen. Patients taking prostanoids or other PDE inhibitors were excluded. The protocol was approved by the ethics committee of Shanghai Tenth People’s Hospital of Tongji University, and written informed consent was obtained from all patients. This trial has been registered with the Shanghai Municipal Public Health Bureau (‘tadalafil and ambrisentan used in patients with PAH,’ no. SH2010-1065).
Study design
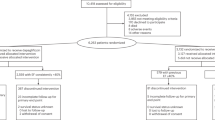
Eligible patients were allocated to add either tadalafil (40 mg per day) or placebo to existing therapy with oral ambrisentan (10 mg per day) in this 16-week double-blind study (Figure 1). The randomization was stratified for baseline walking distance and type of PAH (idiopathic/familial and anorexigen use vs. other types).
Outcome measures
Exercise capacity was evaluated by performing an unencouraged 6MWD at baseline and repeating every 4 weeks until the last day of week 16. Symptom improvements were also assessed by the WHO FC score at baseline and on the last day of week 16. Clinical worsening (CW) was defined as the occurrence of the following events: death, transplantation, arterial septostomy, hospitalization due to worsening PAH, initiation of new therapy or worsening FC by week 16. Hemodynamic parameters were measured by right heart catheterization at baseline and study completion or exit.
Statistical analysis
All randomized patients receiving medications were analyzed for treatment efficacy. The primary analysis for 6MWD was tested with a permutation test on rank. For patients who died or had CW, the lowest rank was applied. For patients who discontinued the study because of treatment-related adverse events, no change in rank was assumed. For all other patients, the change from baseline to the last observed valued was used to assign rank. The treatment difference was also estimated using the analysis of covariance model with a type II sum of squares including terms for randomization factors. Repeated-measures analysis of variance was used to assess the change over time. The hemodynamic parameters at baseline and at trial exit were compared using a paired t-test. The frequency of patients who experienced CW, a change in FC or adverse events was analyzed using Fisher’s exact test. A P-value <0.05 was considered to be statistically significant.
Results
Demographic data
A total of 124 patients were enrolled in this study. Of the 124 patients, 60 patients were randomized to the tadalafil group and 64 were randomized to the placebo group (Figure 1). Patient demographic data are described in Table 1. There were no significant differences between the tadalafil and placebo groups in any demographic or baseline characteristics. Most patients showed typical hemodynamic parameters for moderate to advanced PAH. Eleven patients withdrew from the study prematurely: six in the tadalafil group and five in the placebo group. The hemodynamic profiles of the patients before they began taking tadalafil or placebo are also listed. Overall, the hemodynamic parameters of the patients in the tadalafil group at baseline were slightly better; the mPAP value was slightly higher in the placebo group, while the cardiac output (CO) and PVR values were similar.
Measure of efficacy
The hemodynamic parameters of the patients were measured at the exit of the study and compared with the parameters collected at baseline (Table 2). With oral administration of tadalafil, the mean value of the maximal rate of reduction in mPAP was 14.2 for all patients. When the effect of tadalafil on the mPAP was analyzed in terms of PVR and CO, the mean values of the reduction in PVR and the maximal rate of increase in CO were 26.7% and 15.8%, respectively, for all the patients. Next, the maximal effect was compared between the tadalafil and placebo groups. Although no significance was found in the statistical analysis, the mPAP dropped slightly more in patients taking tadalafil than in those taking placebo. When the PVR and CO values were compared, there was a smaller reduction in PVR in the placebo group and the CO was less improved in the placebo group, explaining the small reduction in the mPAP.
Figure 2 presents the mean change from baseline in the 6MWD at each visit for both the placebo and tadalafil groups. Although patients taking the placebo showed slightly improved exercise capacity from week 8, no statistically significant difference was found between the exercise capacity at baseline and at week 16. This finding can be expected from the study design because all the enrolled patients had been on ambrisentan for at least 4 months and had had a stable 6MWD for at least 1 month. As for the tadalafil group, patients showed absolute improvement in 6MWD from week 4 compared with baseline (not statistically significant), whereas the 6MWD was significantly improved at weeks 8, 12 and 16 (P<0.05). Next, the exercise capacity was compared between the two treatment groups. Better exercise capacity was observed at all visits compared with patients in the control group. However, patients taking tadalafil only showed a significant increase in 6MWD at the trial exit (P=0.042) when compared with the placebo group; these results may indicate that patients in need of dual therapy may benefit from prolonged tadalafil treatment in addition to background ambrisentan.
The 6-min walk distance (6MWD) by visit (tadalafil 40 mg and placebo). Patients took the oral study drug once daily for 16 weeks and a 6-min walk test was performed at baseline and weeks 4, 8, 12 and 16. Values are placebo-adjusted mean values and 95% confidence intervals are presented. The values for each point are presented with variability in brackets. *P<0.05 vs. baseline of tadalafil group, #P<0.05 vs. placebo group at the same time point.
To obtain more information on the therapeutic effect of add-on tadalafil, the changes in the 6MWD of the subgroups were separated by predefined baseline characteristics. As shown in Table 3, no significant differences were found in the demographic characteristics of the patients. In patients with a baseline 6MWD <325 m, combination therapy with tadalafil and ambrisentan showed greater efficacy compared with ambrisentan monotherapy, whereas no significant difference was found between the tadalafil group and the placebo group. The changes in 6MWD in patients in different FCs were also investigated. Patients in FC I–II received greater therapeutic benefit when tadalafil was administered as an add-on to background ambrisentan therapy (not statistically significant). Although improvements in exercise capacity were also found in patients in FC III–IV, the improvement was not statistically significant compared with the placebo group. Next, the change in exercise capacity was analyzed in subgroups with different durations of PAH; the results indicated that significant improvement was only observed in patients who had PAH for <2 years.
The changes in FC are presented in Table 4, and the results were analyzed using Fisher’s exact test. Although the results suggested a greater numeric improvement in FC in the tadalafil group compared with the placebo group, this improvement had no clinical significance (P=0.195).
The results of another clinical marker of efficacy, CW, are presented in Table 5. Across the two treatment groups, the placebo and tadalafil groups, numeric differences were found in the number of patients who worsened during the trial. CW was found in 14 patients in the placebo group and 5 patients in the tadalafil group, suggesting that tadalafil add-on led to a significant delay in CW (P=0.046).
Safety
There were no clinically significant changes in pulmonary function tests, chest radiography or clinical laboratory parameters, including blood chemistries, hematology and coagulation times, between treatment groups. Tadalafil was generally well tolerated by patients, with the most common adverse events being mild-to-moderate headache, flushing and blurred vision. All reported adverse events are listed in Table 6. No significant differences in treatment emergent adverse events or the overall incidence of adverse events were observed. Headache was the most common adverse event in both groups and the majority of headaches were tolerated. Three patients in the tadalafil group dropped out due to toxicity; two patients with migraine histories dropped out due to headache and one patient withdrew due to hypotension and a syncopal episode. In total, there was a similar proportion of adverse events reported, although slightly fewer serious adverse events were reported in the placebo group than in the tadalafil group, which could be the expected result for a more complex therapeutic strategy.
Discussion
Evidence-based guidelines recommend starting treatment in patients with newly diagnosed PAH with monotherapy.19, 20 The American College of Cardiology, the American Heart Association and the European Society of Cardiology recommend using sildenafil, ambrisentan or bosentan as first-line treatments for patients with WHO FC III.21 Combination therapy is recommended as second-line treatment for those patients who do not respond to the initial treatment or those who improve initially with monotherapy without sustained long-term therapeutic success. In addition, patients with a 6MWD of <380 m, signs of right-sided heart failure and persistent FC III or IV symptoms unresponsive to single-agent therapy can also benefit from combination therapy.22, 23
Currently, combination therapy with bosentan and a PDE-5 inhibitor (sildenafil or tadalafil) is the most widely used combination therapy.24, 25 The co-administration of bosentan and tadalafil was well tolerated. However, prolonged use of bosentan reportedly induces the expression of CYP3A4 and CYP2C9, while tadalafil is metabolized by CYP3A4.26 Therefore, possible pharmacokinetic interactions between the two agents may compromise the therapeutic benefit. Wrishiko’s study showed that tadalafil exposure was reduced by 41.5% after 10 days of the co-administration of bosentan, whereas no clinically relevant difference (<20%) in bosentan exposure was found. Ambrisentan is a selective ET-A antagonist with clinical effects similar to those of bosentan and without any influence on CYP3A4 (ambrisentan itself is a substrate of CYP3A4 and CYP2C9).27 This has led to interest in the clinical application of ambrisentan and tadalafil as combination therapy.
In addition, a recent study by Liang et al.25 showed that ambrisentan and tadalafil synergistically relaxed the ET-constricted pulmonary arteries. However, no clinical data regarding the use of the combination therapy of ambrisentan and tadalafil have been reported. To date, this study is the largest randomized, double-blind, placebo-controlled trial where the safety and efficacy of tadalafil as an add-on to background ambrisentan were evaluated. This study contributed to a better understanding of the combination therapy of these two drugs. Moreover, efficacy analyses of patients in the subgroups separated by baseline parameters may help characterize the best candidates for combination therapy with ambrisentan and tadalafil.
In the one other placebo-controlled combined therapy trial that reported the use of tadalafil as an add-on to another ET-1 antagonist, bosentan, Barst et al.20 randomized PAH patients treated with background bosentan to receive tadalafil (20 or 40 mg) or placebo. Improvements in exercise capacity, FC and CW were observed in the tadalafil 40-mg group. However, the improvements were not supported by statistical analyses. No significant increase in adverse events was found, confirming the safety of the combination therapy. As the first placebo-controlled study to evaluate the efficacy and safety of tadalafil as an add-on therapy to a background ET-1 antagonist, their data were not sufficient to conclude the presence of additional therapeutic benefit.
In this study, 124 patients with confirmed PAH who had received background ambrisentan for at least 4 months were randomized to receive oral tadalafil (40 mg) or placebo. The patients who received the dual therapy showed improvement in the 6MWD and fewer cases of CW (P<0.05 vs. placebo). FC was improved in 43.3% of patients in the tadalafil group and 31.3% of placebo patients; however, this difference was not statistically significant (P<0.05). Consistent with these clinical benefits, patients taking oral tadalafil also showed improved cardiopulmonary hemodynamics including PAP, PVR and CO, although these parameters were not significantly improved compared with the placebo group. A previous study demonstrated that tadalafil and ambrisentan were well tolerated and safe. No concerns about pharmacokinetic interactions have been raised.18 Consistent with previous studies, this study also showed that the combination therapy appeared safe and well tolerated; most adverse events were mild to moderate. Overall, patients may benefit from add-on tadalafil for improving exercise capacity and delaying disease progression.
In this study, all patients had been on ambrisentan for at least 4 months and had a stable 6MWD and FC for at least 1 month before enrollment. Therefore, by comparing the data from the combined therapy group with the placebo group, the additional efficacy benefit of tadalafil could be found. However, whether 1 month of stable 6MWD and FC is sufficient to define the stability of the patient remains unresolved.
Although ambrisentan and tadalafil showed synergistic effects in relaxing ET-contracted pulmonary rings in an animal experiment,25 our present study could not determine whether tadalafil offers additive or synergistic effects to background ambrisentan because of the lack of the third arm in which tadalafil replaces ambrisentan rather than is added to it. Owing to the chronic inhibitory effect of ambrisentan on disease activity (for example, through pulmonary artery remodeling), ambrisentan therapy itself may lead to improvements in efficacy, which will interfere with the data interpretation. In current clinical practice, tadalafil tends to be added in patients who have failed or did not receive sufficient therapeutic benefit from standard therapy, rather than as a replacement. Nevertheless, the formal evaluation of the withdrawal of ambrisentan at the time of the initiation of tadalafil may have important clinical implications and will help to further evaluate the necessity of combination therapy, which should be considered in designing further studies.
In addition to the limitations imposed by the selection of study patients and the study design, a number of questions remain unclear: it is unknown whether combination therapy could provide long-term therapeutic benefits, and it is unknown whether similar benefits could be achieved in the case of the concurrent initiation of the two drugs in treatment-naive subjects because the findings of this study are related to the ‘sequential’ addition of tadalafil to patients who were already being treated with ambrisentan.
On the basis of our results overall, tadalafil 40 mg daily appears to be well tolerated and safe when added to background ambrisentan. Combined therapy significantly improved the exercise capacity and CW, although no significant differences in hemodynamic parameters were observed. However, owing to limitations of this study, the data from this study are insufficient to conclude the superiority of combination therapy over monotherapy. Large-scale and long-term studies with more strict patient selection criteria are needed to fully evaluate the therapeutic efficacy of the combination of tadalafil and ambrisentan.
References
Rubin LJ . Primary pulmonary hypertension. New Engl J Med 1997; 336: 111–117.
Galie N, Manes A, Branzi A . [New insights on pulmonary arterial hypertension]. Revista Espanola de Cardiologia 2004; 57: 603–607.
D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT . Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349.
Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ . Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet 2001; 358: 1119–1123.
Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, Marumo F . Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest 1993; 91: 1367–1373.
Fujitani Y, Ueda H, Okada T, Urade Y, Karaki H . A selective agonist of endothelin type B receptor, IRL 1620, stimulates cyclic GMP increase via nitric oxide formation in rat aorta. J Pharmacol Exp Ther 1993; 267: 683–689.
Galie N, Rubin L, Hoeper M, Jansa P, Al-Hiti H, Meyer G, Chiossi E, Kusic-Pajic A, Simonneau G . Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 2008; 371: 2093–2100.
Akagi S, Matsubara H, Miyaji K, Ikeda E, Dan K, Tokunaga N, Hisamatsu K, Munemasa M, Fujimoto Y, Ohe T . Additional effects of bosentan in patients with idiopathic pulmonary arterial hypertension already treated with high-dose epoprostenol. Circ J 2008; 72: 1142–1146.
Humbert M, Barst RJ, Robbins IM, Channick RN, Galiè N, Boonstra A, Rubin LJ, Horn EM, Manes A, Simonneau G . Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J 2004; 24: 353–359.
Seyfarth HJ, Pankau H, Hammerschmidt S, Schauer J, Wirtz H, Winkler J . Bosentan improves exercise tolerance and Tei index in patients with pulmonary hypertension and prostanoid therapy. Chest 2005; 128: 709–713.
McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, Badesch DB, Barst RJ, Hsu HH, Rubin LJ . Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006; 174: 1257–1263.
Simonneau G, Rubin LJ, Galie N, Barst RJ, Fleming TR, Frost AE, Engel PJ, Kramer MR, Burgess G, Collings L, Cossons N, Sitbon O, Badesch DB, PACES Study Group. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med 2008; 149: 521–530.
Hoeper MM, Leuchte H, Halank M, Wilkens H, Meyer FJ, Seyfarth HJ, Wensel R, Ripken F, Bremer H, Kluge S, Hoeffken G, Behr J . Combining inhaled iloprost with bosentan in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2006; 28: 691–694.
McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H, Rubenfire M, Seeger W . Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol 2010; 55: 1915–1922.
Benza RL, Rayburn BK, Tallaj JA, Pamboukian SV, Bourge RC . Treprostinil-based therapy in the treatment of moderate-to-severe pulmonary arterial hypertension: long-term efficacy and combination with bosentan. Chest 2008; 134: 139–145.
Kemp K, Savale L, O’Callaghan DS, Jaïs X, Montani D, Humbert M, Simonneau G, Sitbon O . Usefulness of first-line combination therapy with epoprostenol and bosentan in pulmonary arterial hypertension: an observational study. J Heart Lung Transplant 2012; 31: 150–158.
Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, Badesch DB, Frost AE, Shapiro SM, Laliberte K, Sigman J, Arneson C, Galiè N . Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest 2012; 142: 1383–1390.
Spence R, Mandagere A, Harrison B, Dufton C, Boinpally R . No clinically relevant pharmacokinetic and safety interactions of ambrisentan in combination with tadalafil in healthy volunteers. J Pharm Sci 2009; 98: 4962–4974.
Anderson JR, Nawarskas JJ . Pharmacotherapeutic management of pulmonary arterial hypertension. Cardiol Rev 2010; 18: 148–162.
Barst RJ, Oudiz RJ, Beardsworth A, Brundage BH, Simonneau G, Ghofrani HA, Sundin DP, Galiè N, Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group. Tadalafil monotherapy and as add-on to background bosentan in patients with pulmonary arterial hypertension. J Heart Lung Transplant 2011; 30: 632–643.
Maki H, Yao A, Inaba T, Shiga T, Hatano M, Kinugawa K, Yamashita T, Aizawa T, Nagai R . Initial and programmed combination therapy with oral drugs for severe idiopathic pulmonary arterial hypertension. Int Heart J 2011; 52: 323–326.
Chin KM, Rubin LJ . Pulmonary arterial hypertension. J Am Coll Cardiol 2008; 51: 1527–1538.
Hoeper MM, Markevych I, Spiekerkoetter E, Welte T, Niedermeyer J . Goal-oriented treatment and combination therapy for pulmonary arterial hypertension. Eur Respir J 2005; 26: 858–863.
Sidorenko BA, Preobrazhenskii DV, Batyraliev TA, Belenkov IuN . [Pulmonary arterial hypertension: changing approaches to management]. Kardiologiia 2011; 51: 100–108.
Liang F, Yang S, Yao L, Belardinelli L, Shryock J . Ambrisentan and tadalafil synergistically relax endothelin-induced contraction of rat pulmonary arteries. Hypertension 2012; 59: 705–711.
Wrishko RE, Dingemanse J, Yu A, Darstein C, Phillips DL, Mitchell MI . Pharmacokinetic interaction between tadalafil and bosentan in healthy male subjects. J Clin Pharmacol 2008; 48: 610–618.
Yao A . Recent advances and future perspectives in therapeutic strategies for pulmonary arterial hypertension. J Cardiol 2012; 60: 344–349.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhuang, Y., Jiang, B., Gao, H. et al. Randomized study of adding tadalafil to existing ambrisentan in pulmonary arterial hypertension. Hypertens Res 37, 507–512 (2014). https://doi.org/10.1038/hr.2014.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2014.28
Keywords
This article is cited by
-
Future Perspectives of Pulmonary Arterial Hypertension: A Review of Novel Pipeline Treatments and Indications
Drugs in R&D (2024)
-
Effect of Combination Therapy of Endothelin Receptor Antagonist and Phosphodiesterase-5 Inhibitor on Clinical Outcome and Pulmonary Haemodynamics in Patients with Pulmonary Arterial Hypertension: A Meta-Analysis
Clinical Drug Investigation (2019)
-
Ambrisentan ± tadalafil in WHO functional class II/III pulmonary arterial hypertension: a guide to its use in the EU
Drugs & Therapy Perspectives (2018)
-
Sildenafil dosed concomitantly with bosentan for adult pulmonary arterial hypertension in a randomized controlled trial
BMC Cardiovascular Disorders (2017)
-
Ambrisentan in pulmonary arterial hypertension: a guide to its use in the EU
Drugs & Therapy Perspectives (2016)