Abstract
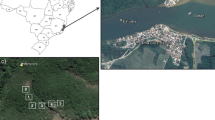
We report the results of carbon stored in soil and aboveground biomass from the most important area of mangroves in Mexico, with dominant vegetation of Red mangrove (Rhizophora mangle L.), Black mangrove (Avicennia germinans L.), white mangrove (Laguncularia racemosa Gaertn.) and button mangrove (Conocarpus erectus L.). We sampled soils with high fertility during the dry season in 2009 and 2010 at three sites on Atasta Peninsula, Campeche. We used allometric equations to estimate above ground biomass (AGB) of trees. AGB was higher in C. erectus (253.18±32.17 t·ha−1), lower in A. germinans (161.93±12.63 t·ha−1), and intermediate in R. mangle (181.70±16.58 t·ha−1) and L. racemosa (206.07±19.12 t·ha−1). Of the three studied sites, the highest absolute value for AGB was 279.72 t·ha−1 in button mangrove forest at any single site. Carbon stored in soil at the three sites ranged from 36.80±10.27 to 235.77±66.11 t·ha−1. The Tukey test (p <0.05) made for AGB was higher for black mangrove showed significant differences in soil carbon content between black mangrove and button mangrove. C. erectus had higher AGB compared with the other species. A. germinans trees had lower AGB because they grew in hypersaline environments, which reduced their development. C. erectus grew on higher ground where soils were richer in nutrients. AGB tended to be low in areas near the sea and increased with distance from the coast. A. germinans usually grew on recently deposited sediments. We assumed that all sites have the same potential to store carbon in soil, and then we found that there were no significant differences in carbon content between the three samples sites: all sites had potential to store carbon for long periods. Carbon storage at the three sampling sites in the state of Campeche, Mexico, was higher than that reported for other locations.
Similar content being viewed by others
References
Amarasinghe MD, Balasubramaniam S. 1992. Net primary productivity of two mangrove forest stands on the northwest coast of Sri Lanka. Hydrobiology, 247: 37–47.
Arreaga W. 2002. Carbon storage in forest with a management program in the natural reserve “Maya Peten”, Guatemala. Thesis M. Sc. CATIE, Turrialba, CR. p.86.
Baccini A, Laporte N, Goetz S J, Sun M, Dong H. 2008. A first map of tropical Africa’s above-ground biomass derived from satellite imagery. Environmental Research Letters 3(4): 1–9.
Basuki TM, Van Laake PE, Skidmore AK, Hussin Y A. 2009. Allometric equations for estimating the above-ground biomass in tropical lowland Dipterocarp forest. Forest Ecology and Management, 257: 1684–1694.
Bridgham SD, Megonigal JP, Keller JK, Bliss NB, Trettin C. 2006. The carbon balance of North American wetlands. Wetlands, 26: 889–916.
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Folster H, Fromard F, Higuchi N, Kira T, Lescure JP, Nelson BW, Ogawa H, Puig H, Rie’ra B, Yamakura T. 2005. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia, 145: 87–99.
Craft CB, Seneca ED, Broome SW. 1991. Ignition and kjeldahl digestion for estimating organic carbon and soils: Calibration with dry combustion. Estuaries, 14: 175–179.
Day JW, Conner WH, Ley LF, Day RH, Navarro AM. 1987. The productivity and composition of mangrove forests, Laguna de Terminos, Mexico. Aquatic Botany, 27: 267–284.
Fromard F, Puig H, Mougin E, Marty G, Betoulle JL, Cadamuro L. 1998. Structure above-ground biomass and dynamics of mangrove ecosystems: new data from French Guiana. Oecologia, 115:39–53.
Gonzalez M, Etchevers B, Hidalgo M. 2008. Carbono en suelos de ladera: factores que deben considerarse para determinar su cambio en el tiempo. Agrociencia, 42(7): 741–751.
Hawes JE, Peres CA, Riley LB, Hess LL. 2012. Landscape-scale variation in structure and biomass of Amazonian seasonally flooded and unflooded forests. Forest Ecology and Management, 281: 163–176.
Heiri O, Lotter AF, Lemcke G. 2001. Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. Journal of paleolimnology, 25:101–110.
Instituto Nacional de Ecología. 1997. Programa de Manejo del área de protección de flora y fauna “Laguna de Términos”; SEMARNAT. México. p.167.
IPCC (Intergovernmental Panel on Climate Change). 2001. Climate Change: The Scientific Basis. Cambridge, UK: Cambridge Univ. Press, p.881.
Komiyama A, Ong JE, Poungparn S. 2008. Allometry, biomass, and productivity of mangrove forests: a review. Aquatic Botany, 89: 128–137.
Komiyama A, Poungparn S, Kato S. 2005. Common allometric equations for estimating the tree weight of mangroves. Journal of Tropical Ecology, 21: 471–477.
Lugo A, Snedaker C. 1974. The ecology of mangroves. Annual Review of Ecology and Systematics, 5: 38–64.
Mall LP, Singh VP, Garge A. 1991. Study of biomass, litter fall, litter decomposition and soil respiration in monogeneric mangrove and mixed mangrove forest of Andaman Island. Tropical Ecology, 32: 144–152.
Ordoñez JA, Masera O. 2001. Captura de carbono ante el cambio climático. Madera y Bosques, 7: 3–12.
Ordoñez JA, Bernardus HJ, Masera O. 2001. Almacenamiento de carbono en un bosque de Pinus pseudostrobus en Nuevo San Juan Michoacan Madera y Bosques, 7(2): 27–47
Rico-Gray V. 1982. Estudio de la vegetación de la zona costera inundable del noroeste de Campeche, México: Los petenes. Biótica, 7: 171–188.
Twilley RW, Lugo AE, Patterson-Zucca C. 1986. Litter production and turnover in basin mangrove forests in Southwest Florida. Ecology, 67: 670–683.
Webb A. 2002. Pre-clearing soil carbon levels in Australia. National carbon accounting system technical report No.12. Australian greenhouse office, Canberra, 204.
Whiting JG, Chanton JP. 2001. Greenhouse carbon balance of wetlands: methane emission versus carbon sequestration. Tellus, 53B: 521–528.
Zanne AE, Lopez GG, Coomes DA, Llic J, Jansen SL, Lewis SL, Miller RB, Swenson NG, Wiemann MC, Chave J. 2009. Global wood density database. Dryad. Identifier: http://hdl.handle.net/10255/dryad.235. August 4th 2012
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guerra-Santos, J.J., Cerón-Bretón, R.M., Cerón-Bretón, J.G. et al. Estimation of the carbon pool in soil and above-ground biomass within mangrove forests in Southeast Mexico using allometric equations. Journal of Forestry Research 25, 129–134 (2014). https://doi.org/10.1007/s11676-014-0437-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-014-0437-2