Abstract
Background
Recombinant human growth hormone (rhGH) can increase the growth rate in growth hormone deficient children (GHD). In this randomized clinical trial, we compared the efficacy and side effects of an Iranian brand; Samtropin with Norditropin.
Methods
The GHD children were randomly treated either with standard dose of Samtropin or Norditropin rhGH for one year. Upstanding height, height standard deviation score (HSDS), growth velocity (GV), serum levels of insulin like growth factor-1 (IGF-1), and bone age (BA) were determined before and during one year treatment concomitant side effects of treatment.
Results
We evaluated 22 subjects; 12 on Samtropin and, 10 on Norditropin. In each group, mean age was 12 yr and 50% of them were male. The mean differences in height, HSDS, IGF-1 and BA by Norditropin before and after 12 months were 8.8 cm, 0.5, 49 ng/ml and 2.8 yr, respectively. These measures by Samtropin were 9.1 cm, 0.6, 133 ng/ml, and 1.7 yr, respectively without any significant difference. The mean of GV by Samtropin was 9.1 vs. 8.8 cm by Norditropin without significant difference. Since the efficacy of Samtropin was found to be similar to Norditropin after 12 months; we switched to use only Samtropin for the next 12 months. The mean differences in height, HSDS, GV and BA in 20 children between months 12 and 24 were 7.0 cm, 1.6, 2.1 cm/yr and 1.0 yr, respectively (P < 0.001). We also found a non-significant decrease in IGF-1 levels. No side effects were observed.
Conclusions
We need to conduct a post marketing surveillance with a large sample size in order to confirm our findings.
Trial registration
Registration code number in the Iranian Registry of Clinical Trials (IRCT): IRCT1138901181414N11.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recombinant human growth hormone (rhGH) has been established as an appropriate treatment to increase the growth rate in children with growth hormone deficiency (GHD), Turner syndrome (TS), Prader-Willi syndrome (PWS), chronic renal failure (CRF), and being born small for gestational age (SGA) [1]. Considering the large heterogeneity in diagnosis of GHD in children, its prevalence and incidence is largely varied in different countries [2],[3]. However, its appropriate treatment is important, because these children are at higher risk of social isolation [4], anxiety and poor school performance [5], cardiovascular morbidity [6], type 2 diabetes mellitus and metabolic syndrome [7],[8].
It is shown that rhGH could improve the linear growth partly through stimulation of the production of insulin like growth factor-1 (IGF-1) [9]. IGF-1 has insulin like effects, could inhibit lipolysis and activate bone remodeling [9].
The rhGH is derived from recombinant DNA and is produced by several pharmaceutical companies such as Genentech, NOVO Nordisk, Serono, etc. [10]. All of these products have equivalent therapeutic efficacy and pharmacokinetic properties [11]. Since the cost of GH therapy is substantial; it considers as an important issue for medical resources allocation. Given the current treatment costs in the United States, this correspondence to more than 50,000 $ USD per inch (2.54 cm) gained in adult height; which is a significant expense for any health system [12]. To lower the cost, one solution would be the production of rhGH by several pharmaceutical companies, especially domestic companies. Recently, Samtropin rhGH has been produced by an Iranian Pharmaceutical Company; Samen. However to establish its efficacy and safety, we should compare it with a Food and Drug Administration (FDA) approved rhGH. The aim of the present study was to demonstrate the safety and efficacy of Samtropin in comparison with Norditropin; a product that is manufactured by NOVO Nordisk Company to treat the linear growth failure in pediatric GHD. By performing this study we may be able to present the Samtropin rhGH as a less expensive drug for treatment of GHD children.
Materials & methods
Study population
This parallel non-inferiority/equivalence randomized clinical trial (RCT) study was carried out from August 2006 to April 2011 to compare the efficacy and side effects of Iranian brand of rhGH; Samtropin (Samen Company) to Norditropin (NOVO Nordisk Company) in pediatric GHD. Before starting the study, informed consent was obtained from all eligible children. GHD in children was diagnosed by failure to reach serum GH levels ≥10 ng/ml by clonidine GH-stimulation test] [13]. Inclusion criteria of the study was defined as non-pubertal children who have height standard deviation score (HSDS) < −3SD, or short stature children who have concomitant level of IGF-1 below that of children of the same chronological age and sex recorded for IGF-1 kit. Exclusion criteria were set as: other causes of GHD such as hypothyroidism, celiac, any acute or systemic infection diseases, seizure, HIV disorder, any chronic systemic disorders such as diabetes mellitus or CRF, TS, sleep apnea syndrome, active cancers, acute phase of craniopharengioma or other contraindications of GH replacement therapy, and concomitant use of corticosteroid.
This study included two phases. In the first phase; patients were divided in a random double blind manner into two groups; intervention group who received Samtropin and control group who received Norditropin. Both groups received the treatment over a period of 12 months, as a bed time subcutaneous injection at dose of 0.04 mg (0.1 IU)/Kgw/day, with maximum dose of 4 IU/day. We used same form of rhGH (vial) with similar color and package with a lable. The main researcher and patients were blinded to the interventions. The lable was a code cleared only for the third adviser. All the enrolled children were visited by a physician every month for the first 6 months, and then at 12th month after starting treatment to assess response to rhGH treatment. The assessed parameters were height, HSDS, growth velocity (GV), weight standard deviation score (WSDS), bone age (BA), and serum levels of IGF-1. GV was determined according to below formula:
Parents’ height was assessed before initiating the study to predict the height potential of children (PHP). We used the cross-sectional data provided by the National Center for Health Statistics (NCHS) from the Centers for Diseases Control (CDC) web site to estimate HSDS and WSDS [14]. Pubertal status was assessed and scored at each visit according to Marshall-Tunner staging [15]. Bone age was determined before and at the end of one year treatment.
Safety of the treatment was assessed by monitoring the adverse effects, measuring the body weight, performing the laboratory tests, and fundoscopy. Adverse effects were assessed in each visit according to a questionnaire consisting these signs; headache, edema, arthralgia, vomiting, hypoglycemia, genicomastia, lipoathrophia or lipohyperthrophia or local reaction in injection site, carpal tunnel syndrome, obesity, vertigo, seizure, recent visual deficit and muscle cramp. Laboratory tests included the routine hematology tests, blood chemistry, glucose metabolism (fasting blood sugar), and thyroid hormone; free thyroxin (FT4). Fundoscopy was performed at the baseline for all the patients and during the course of study forpatients who developed headache or signs of intracranial hypertension.
At the end of first year, we analyzed the findings and we found similarity in the therapeutic effects of these two brands; height, HSDS, GV, BA and IGF-1. Based on this finding, the second phase of the study was designed to examine the Samtropin’s therapy impact on growth parameters. To do this, Samtropin therapy was continued for the next 12 months for all the patients, and height and HSDS were measured at 18th, and 24th month of the study. Moreover, probable side effects and puberty status were assessed at the above time points according to the same criteria of the first phase. Bone age, IGF-1, glucose metabolism and thyroid hormone level were determined at the end of the second year of the study.
The study was approved by ethical committee of Endocrinology and Metabolism Research Center (EMRC) of Endocrinology and Metabolism Clinical Sciences Institute of Tehran University of Medical Sciences (TUMS) and registered in the Iranian Registry of Clinical Trials (IRCT) as code number of IRCT1138901181414N11.
Laboratory methods
Fasting blood samples were obtained from all subjects to evaluate below biochemical measures; CBC-diff, blood urea nitrogen (BUN), creatinine (Cr), alkaline phosphates (Alkp), alanine aminotransferase (ALT), aspartate aminotransferase (AST), fasting blood sugar (FBS), sodium (Na), potassium (K), FT4, and IGF-1. FBS and BUN were measured by enzymatic method, using Pars- Azmon kit/Iran. Cr was measured by JAFFE method (Pars- Azmon kit/Iran). Alkp, ALT, and AST were measured using photometer assay, enzymatic method. Na and K were measured by flame photometer Coring 480/US. CBC-diff was assessed by cell counter ABACUS. FT4 was measured by ELISA method using monobind kit/USA. IGF-1 was measured by RIA method using Immunotech kit/Chekslovaki, ranging 76–499 ng/ml for children aged more than 4 years.
Statistical analysis
The normal distribution of data was evaluated by Kolmogrov-Smirnov analytic test. After determining the mean of variables, the student two-tailed t test was applied to compare the mean differences. Repeated measures were used to compare the trend of variables in different times. SPSS software (version 16) was used to perform the statistical analysis and p-values ≤ 0.05 were considered statistically significant.
Results
The first phase
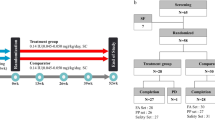
From 45 initially evaluated subjects, after considering inclusion and exclusion criteria, 22 patients were eligible to be enrolled in the study. Flow diagram of the study is shown in Figure 1.
The baseline characteristics of 22 patients are summarized in Table 1 (10 children in NOVO group as control group, and 12 children in Samen group as intervention group). The mean age in both groups was similar (12 yr). In each group, 50% of the participants were male and most of them were in non-pubertal status. There were no significant differences in baseline characteristics of patients between two groups. All the children except one, who was undertreatment with levothyroxin, had normal FT4 levels.
The mean difference of height, HSDS, and IGF-1 produced by Norditropin after 12 months of treatment were 8.8 cm, 0.5, and 49 ng/ml, respectively, while these measures by Samtropin were 9.1 cm, 0.6, and 133 ng/ml, respectively. No significant difference was detected between groups (Table 2). Figures 2, 3 and 4 show the trend of change in height, HSDS and IGF-1 during 12 months treatment with NOVO or Samen rhGH.
The mean of WSDS in NOVO group at baseline and 12 months after start of therapy was −2.3 and −1.9, respectively, without statistically significant difference within group (p = 0.20). These figures within Samen group were −2.3 and −1.6, respectively (p = 0.009). There was no significant difference in mean difference of WSDS after 12 months rhGH therapy between groups (p = 0.22). Mean of BA from a baseline value of 8.7 year increased to 11.6 year at month 12 (P < 0.001) in NOVO group and from 9.6 year to 11.3 year (p = 0.004) in Samen group. The mean difference of BA after 1 year treatment with Norditropin rhGH was 2.8 year and with Samtropin rhGH was 1.7 (p = 0.11).
The mean of PHP for NOVO group was 159.3 cm and its difference with the baseline height of this group was 30.4 cm (P < 0.001). This difference changed to 21.7 cm at month 12 (P < 0.001). The mean of PHP in Samen group was 162.1 cm and its difference with the baseline height of this group was 29.3 cm (P < 0.001). The measure changed to a mean of 20.2 cm at month 12 (P < 0.001). No remarkable difference in mean difference height from PHP after one year treatment between two groups was detected (p = 0.74). The mean of GV induced by Samtropin rhGH was similar to Norditropin; 9.1 vs. 8.8 cm, respectively without significant difference.
Neither life-threatening adverse events nor significant changes in biochemical tests were reported during the study in both groups.
The second phase
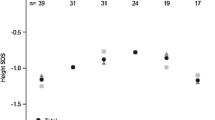
Since the efficacy of Samtropin rhGH was similar to Norditropin after 12 months treatment; we switched to use only Samtropin in all children for the next 12 months. Within 22 patients entered the second phase of the study, 20 children completed this phase. In this phase, we considered 12th month as baseline and 24th month as the end of study’s second phase. The mean of Height, HSDS and GV at the 12th month of the study were 140.1 cm, −2.5 and 9.1 (Table 3). These figures changed to 147.1 cm, −0.9, and 7.0, respectively at the 24th month of the study (p < 0.001).
Figures 5, 6 and 7 demonstrate the trends of height, HSDS and GV during 12 months treatment in the second phase of study.
The mean of WSDS and BA at baseline of the second phase was −1.8 ± 1.5 and 11.4 ± 1.9 yr, respectively, which was improved significantly to 0.6 ± 1.3 and 12.4 ± 2.0 yr (p < 0.001 and p < 0.05), respectively after 12 months of treatment. A non-significant decrease (p = 0.26) in serum IGF-1 levels was detected between baseline (539 ± 268 ng/ml) and month 24 of the study (429 ± 220 ng/ml). The mean difference in PHP between baseline and month 12 of the study was 20.6. This figure declines to 13.6 cm (P < 0.001) at month 24.
Similar to results reported in the first phase of the study, in the second phase we found neither life-threatening adverse events nor significant changes in laboratory data.
Discussion
Auxological parameters
Height, HSDS, and GV are well established measures of linear growth during rhGH treatment, particularly during the usual pre-pubertal age [16],[17]. In our study we found an improvement in height, HSDS, and GV values which could be assumed as the efficient growth response of the children to Samtropin. Similar to other published studies [18]-[20] for all these auxological parameters, significant differences were observed between values of baseline and month 12 of the study within NOVO or Samen groups. However, no significant difference between these two groups was observed. Moreover, this treatment response was not affected by switching from Norditropin to Samtropin in the second phase of the study.
After one year treatment with Samtropin, height difference was 9.1 cm, a change that waseven more than height gained by Norditropin treatment (8.8 cm). It is generally accepted that an increase of at least 0.25 SD in HSDS over the first year of treatment is a definitive evidence of rhGH efficacy [16]. The increase in HSDS after treatment with rhGH therapy in our study was 0.57 by Samtropin and 0.52 by Norditropin without any significant difference between them. When we considered the two years treatment by rhGH in our patients regardless of the brand employed, we found the significant change in HSDS from −2.5 at baseline to −0.9, a figure that is in agreement with other studies [21]-[23]. Root et al. [21] reported that HSDS increased from a mean value of −3.4 to −1.5 after 4 years treatment with Omnitrope in 140 GHD children. Coste et al. [22] reported in French GHD children a HSDS of −2.0 compared with a HSDS of −1.3 after 4 years of treatment with Omnitrope. These figures changed from −2.2 at baseline to −0.9 after 4 years treatment with Omnitrope in British children [23].
The standard curves of height could be affected by the pubertal growth spurt. On the other hand, in children with delayed puberty the changes in HSDS can be masked by using rhGH. Therefore, it seems that GV value may be a more appropriate measure of rhGH response in such children. Inboth our groups, the mean GV increased equally (9.1 vs. 8.8 cm/year), after 12 months of treatment with rhGH. The mean GV increased equally again in both groups (2.1 cm/year) in the second phase of study. Similar to findings of the other published studies [19],[22],[24],[25] the greatest acceleration in GV was occurred during the first year of the rhGH treatment in our study.
Pharmacodynamic parameter
IGF-1 is defined as the preferred pharmacodynamic marker for assessing theactivity of rhGH in similar medicinal products containing somatropin [26]. IGF-1 levels tend to be low in GHD and rise in a dose-dependent manner in children treated with rhGH [27]. Scire et al. [28] demonstrated that at a conventional rhGH dose (0.03 mg/kg/day) abnormal elevation of IGF-1 in GHD children does not occur. In our study, Samtropin similar to Norditropin stimulated the synthesis of IGF-1 which was detected by significant increase in IGF-1 serum level after 12 months of treatment. The increase was more pronounced during the first 6 month of treatment, the time period when the growth of patients was observed to be most accelerated. The increase in IGF-1 level from baseline to which achieved after 12 months treatment with Samtropin in our study was very similar to other studies [18],[29]. The mean level of IGF-1 was decreased non-significantly in the second phase of study.
PHP and bone maturation
The PHP corresponds to the height that patient is expected to reach at the end of linear growth. In our study, the mean difference of PHP and the height of children after 1 year treatment with rhGH in NOVO group was 30.6 cm and for Samen group was 29.3 cm, without any significant difference between groups. When we continued the rhGH therapy with Samtropin for the second year, the mean difference between PHP and the height of children was 13.63 cm. It is shown that responses to rhGH could be influenced by several factors such as dosages of rhGH, degree of delay in growth and skeletal age before initial treatment, and manipulation of puberty [30]. Before starting treatment, we had 3 children who were in pubertal stage; one child in NOVO group and 2 children in Samen group. The number of pubertal kids increased to 11 subjects at the end of 2 years. This fact could lead to variation in response to rhGH particularly in the second year of the study.
After 1 year treatment with Samtropin the bone age of our patients increased by 1.71 year, whilst this figure for Norditropin was 2.85 year. Although the effect of Samtropin on bone age was lower than the effect of Norditropin, there was not any significant difference between treatment groups. The effect of Samtropin on the bone age in the second phase was equal to 1 year. The effect of Samtropin on bone age for the second year was equal to the chronological age. This finding is important since itshows the normal pace of bone maturation, and also illustrates that skeletal growth induced by Samtropin is similar to other rhGH products including Omnitrope and Saizen [18],[20]. Similar to our other findings, the most pronounced effect of Samtropin was produced in the first year of treatment. In the other word, the potential gain in height decreased as the treatment period was increased.
Safety
Mean values of blood chemistry were within normal ranges during study. Monitoring of free T4 and TSH is recommended for detecting hypothyroidism which may appear during rhGH therapy [31],[32]. None of our studied children developed low FT4 while were on rhGH therapy. The rhGH therapy may induce carbohydrate intolerance in children with compromised insulin secretion [33]. None of our children in two phases of the study developed glucose intolerance asmeasured by FBS. This finding is in agreement with Aman et al. [34] and Soliman et al. [35] findings who demonstrated that rhGH therapy could not produce any negative effect on FBS. Overall the safety profile of our treatments was similar to findings of other published studies [29],[35] with no significant changes between our groups. All of our safety assessment results were in agreement with other studies that reviewed the short and long term safety of rhGH therapy [36]-[38]. The most frequent adverse effects reported by other products were headache, eosinophilia, hypothyroidism and injection site reactions [19]. Overall the side effects of rhGH therapy are uncommon and reversible after discontinuation or dose reduction of treatment [30].
Limitations of the study
We have to mention that our experiment has some limitations. We did not measure the free IGF-1 or IGF binding protein-3 (IGFBP-3) as a carrier of IGF-1 in serum. Serum level of IGF-1 reflects the 24-hour secretion of GH. However, the general believe is that free IGF-1 is a biologically active component of IGF-1 [29]. Furthermore, the free IGF-1 is controlled by IGFBP-3 [29]. Our small sample size is another limitation. We understood that we need to design a post marketing surveillance with a larger sample size in order to confirm our findings, however the result of this study have implications for clinical practice and health policy.
Conclusions
In conclusion, the clinical comparability between Norditropin and Samtropin demonstrated that Samtropinat a dose of 0.04 mg/kg/day is effective to increase the linear growth, safe and well tolerated both locally and systemically within 12 months treatment of GHD children. Although, the results of this study are supportive of the long term (up to 2 years) benefits of Samtropin rhGH in GHD children, interpretation of the finding should be done with caution since the second phase of our study was not a comparative, randomized trial. Considering that only small number of our patients received Samtropin for the full 2 years, the long term (2 years) treatment with Samtropin was efficient and well tolerated.
Abbreviations
- rhGH:
-
Recombinant human growth hormone
- GHD:
-
Growth hormone deficient
- TS:
-
Turner syndrome
- PWS:
-
Prader-Willi syndrome
- CRF:
-
Renal failure
- SGA:
-
Small for gestational age
- IGF-1:
-
Insulin like growth factor-1
- FDA:
-
Food and Drug Administration
- RCT:
-
Randomized clinical trial
- HSDS:
-
Height standard deviation score
- GV:
-
Growth velocity
- WSDS:
-
Weight standard deviation score
- BA:
-
Bone age
- PHP:
-
Predict height potential
- NCHS:
-
National Center for Health Statistics
- CDC:
-
Centers for Diseases Control
- FT4:
-
Free thyroxin
- EMRC:
-
Endocrinology and Metabolism Research Center
- TUMS:
-
Tehran University of Medical Sciences
- IRCT:
-
Iranian Registry of Clinical Trials
- BUN:
-
Blood urea nitrogen
- Cr:
-
Creatinine
- Alkp:
-
Alkaline phosphates
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- FBS:
-
Fasting blood sugar
- Na):
-
Sodium
- (K:
-
Potassium
- IGFBP-3:
-
IGF binding protein-3
References
Takeda A, Cooper K, Bird A, Baxter L, Frampton GK, Gospodarevskaya E, Welch K, Bryant J: Recombinant human growth hormone for the treatment of growth disorders in children: a systematic review and economic evaluation. HTA 2010, 14: 1–256.
Juul A, Bernasconi S, Clayton PE, Kiess W, DeMuink-Keizer Schrama S: Drugs and Therapeutics Committee of the European Society for Paediatric Endocrinology (ESPE): European audit of current practice in diagnosis and treatment of childhood growth hormone deficiency. Horm Res 2002, 58: 233–241. 10.1159/000066265
GHD/MPHD - Child Growth Foundation (CGF). Available from: http://www.childgrowthfoundation.org/Default.aspx?page=ConditionsGHD Last updated: 2012-2014., GHD/MPHD - Child Growth Foundation (CGF). Available from: Last updated: 2012–2014. http://www.childgrowthfoundation.org/Default.aspx?page=ConditionsGHD
Voss LD, Mulligan J: Bullying at school: are short pupils at risk? Questionnaire study in a cohort. BMJ 2000, 320: 612–613. 10.1136/bmj.320.7235.612
Tanaka T, Cohen P, Clayton PE, Laron Z, Hintz RL, Sizonenko PC: Diagnosis and management of growth hormone deficiency in childhood and adolescence: Part 2: Growth hormone treatment in growth hormone deficient children. Growth Horm IGF Res 2002, 12: 323–341. 10.1016/S1096-6374(02)00045-X
Rosén T, Wilhelmsen L, Bengtsson BA: Altered lipid pattern explains increased cardiovascular mortality in hypopituitary patients with growth hormone deficiency. Clin Endocrinol 1998, 48: 525–526. 10.1046/j.1365-2265.1998.00473.x
Höybye C, Hilding A, Jacobsson H, Thorén M: Metabolic profile and body composition in adults with Prader-Willi syndrome and severe obesity. J Clin Endocrinol Metab 2002, 87: 3590–3597. 10.1210/jcem.87.8.8735
Reinehr T, Kleber M, Toschke A: Small for gestational age (SGA) status is associated with metabolic syndrome in overweight children. Eur J Endocrinol 2009, 160: 579–584. 10.1530/EJE-08-0914
Gharib H, Cook DM, Saenger PH, Bengtsson BA, Feld S, Nippoldt TB, Rodbard HW, Seibel JA, Vance ML, Zimmerman D: American Association of Clinical Endocrinologists Growth Hormone Task Force: American Association of Clinical Endocrinologists medical guidelines for clinical practice for growth hormone use in adults and children–2003 update. Endocr Pract 2003, 9: 64–76. 10.4158/EP.9.1.64
Miller BS: RhGH Safety and Efficacy Update. Adv Pediatr 2011, 58: 207–241. 10.1016/j.yapd.2011.05.001
Strobl JS, Thomas MJ: Human growth hormone. Pharmacol Rev 1994, 46: 1–34.
Lee JM, Davis MM, Clark SJ, Hofer TP, Kemper AR: Estimated cost-effectiveness of growth hormone therapy for idiopathic short stature. Arch PediatrAdolesc Med 2006, 160: 263–269. 10.1001/archpedi.160.3.263
Wilson DM, Frane J: A brief review of the use and utility of growth hormone stimulation testing in the NCGS: do we need to do provocative GH testing? Growth Horm IGF Res 2005, 15(Suppl A):S21-S25. 10.1016/j.ghir.2005.06.005
Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL: Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to 1977 National Center for Health Statistics version. Pediatrics 2002, 109: 45–60. 10.1542/peds.109.1.45
Marshall WA, Tanner JM: Variations in pattern of pubertal changes in girls. Arch Dis Child 1969, 44: 291–303. 10.1136/adc.44.235.291
Wilson TA, Rose SR, Cohen P, Rogol AD, Backeljauw P, Brown R, Hardin DS, Kemp S, Lawson M, Radovick S, Rosenthal S, Silverman L, Speiser P: Update of guidelines for the use of growth hormone in children: the Lawson Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee. J Pediatr 2003, 143: 415–421. 10.1067/S0022-3476(03)00246-4
Growth Hormone Research Society: Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. GH Research Society J Clin Endocrinol Metab 2000, 85: 3990–3993.
Lopez-Siguero J, Victoria Borras Perez M, Balser S, Khan-Boluki J: Long-term safety and efficacy of the recombinant human growth hormone Omnitrope® in the treatment of Spanish growth hormone deficient children: results of a phase III study. Adv Ther 2011, 28: 879–893. 10.1007/s12325-011-0063-8
Romer T, Saenger P, Peter F, Walczak M, LeBouc Y, Khan-Boluki J, Berghout A: Seven years of safety and efficacy of the recombinant human growth hormone Omnitrope® in the treatment of growth hormone deficient children: results of a phase III study. Horm Res 2009, 72: 359–369. 10.1159/000249164
Bercu BB, Murray FT, Frasier SD, Rudlin C, ODea LS, Brentzel J, Hanson B, Landy H: Long-term therapy with human growth hormone (Saizen®) in children with idiopathic and organic growth hormone deficiency. Endocrine 2001, 15: 43–49. 10.1385/ENDO:15:1:043
Root AW, Kemp SF, Rundle AC, Dana K, Attie KM: Effect of long- term recombinant growth hormone therapy in children – the National Cooperative Growth Study, USA, 1985–1994. J Pediatr Endocrinol Metab 1998, 11: 403–412. 10.1515/JPEM.1998.11.3.403
Coste J, Letrait M, Carel JC, Tresca JP, Chatelain P, Rochiccioli P, Chaussain JL, Job JC: Long-term results of growth hormone treatment in France in children of short stature: population, register based study. BMJ 1997, 315: 708–713. 10.1136/bmj.315.7110.708
Tanner JM, Whitehouse H, Takaishi M: Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965, II. Arch Dis Child 1966, 41: 613–635. 10.1136/adc.41.220.613
Cohen P, Bright GM, Rogol AD, Kappelgaard AM, Rosenfeld RG: Effects of dose and gender on the growth and growth factor response to GH in GH-deficient children: implications for efficacy and safety. J Clin Endocrinol Metab 2002, 87: 90–98. 10.1210/jcem.87.1.8150
Finkelstein BS, Imperiale TF, Speroff T, Marrero U, Radcliffe DJ, Cuttler L: Effect of growth hormone therapy on height in children with idiopathic short stature. Arch Pediatr Adolesc Med 2002, 156: 230–240. 10.1001/archpedi.156.3.230
European Medicines Agency-Committee for medicinal products for human use: Annex guideline: nonclinical and clinical issues on similar medicinal products containing somatropin. EMEA/CHMP/94528/05. 22 February 2006. http://www.emea.europa.eu/pdfs/human/biosimilar/9452805en.pdf., European Medicines Agency-Committee for medicinal products for human use: Annex guideline: nonclinical and clinical issues on similar medicinal products containing somatropin. EMEA/CHMP/94528/05. 22 February 2006. . http://www.emea.europa.eu/pdfs/human/biosimilar/9452805en.pdf
Kamp GA, Zwinderman AH, Van Doorn J, Hackeng W, Frölich M, Schönau E, Wit JM: Biochemical markers of growth hormone (GH) sensitivity in children with idiopathic short stature: individual capacity of IGF-I generation after high-dose GH treatment determines the growth response to GH. Clin Endocrinol (Oxf) 2002, 57: 315–325. 10.1046/j.1365-2265.2002.01575.x
Scire G, Del Bianco C, Spadoni GL, Cianfarani S: Growth hormone therapy does not alter the insulin-like growth factor-1/insulin-like growth factor binding protein-3 molar ration in growth hormone-deficient children. J Endocrinol Invest 2008, 31: 153–158. 10.1007/BF03345582
Wacharasindhu S, Aroonparkmongkol S, Srivuthana S: Measurement of IGF-1, IGFBP-3 and free IGF-1 levels by ELISA in growth hormone (GH) deficient children before and after GH replacement. Asian Pac J Allergy Immunol 2002, 20: 155–160.
Allen DB: Growth hormone therapy for short stature: is the benefit worth the burden? Pediatrics 2006, 118: 343–348. 10.1542/peds.2006-0329
Lippe MB, Van Herle AJ, La Franchi SH, Uller PP, Lavin N, Kaplan SA: Reversible hypothyroidism in growth hormone-deficient children treated with human growth hormone. J Clin Endocrinol Metab 1975, 40: 612–618. 10.1210/jcem-40-4-612
Root AW, Snyder PJ, Revzani I, Digforce AM, Utiger RD: Inhibition of thyrotropin-releasing hormone mediated secretion of thyrotropin by human growth hormone. J Clin Endocrinol Metab 1973, 36: 103–107. 10.1210/jcem-36-1-103
Malozowski S, Tanner LA, Wysowski DK, Fleming GA, Stadel BV: Benign intracranial hypertension in children with growth hormone deficiency treated with growth hormone. J Pediatr 1995, 126: 996–999. 10.1016/S0022-3476(95)70232-6
Aman J, Rosberg S, AJbertsson-Wikland K: Effect of growth hormone treatment on insulin secretion and glucose metabolism in prepubertal boys with short stature. Eur J Endocrinol 1994, 131: 246–250. 10.1530/eje.0.1310246
Soliman AT, AbdulKhadir MM: Growth parameters and predictors of growth in short children with and without growth hormone (GH) deficiency treated with human GH: a randomized controlled study. J Trop Pediatr 1996, 42: 281–286. 10.1093/tropej/42.5.281
EMEA: NutropinAq®: European Public Assessment Report (scientific discussion, 16.07.2008).http://www.emea.europa.eu/humandocs/PDFs/EPAR/nutropinaq/437400en6.pdf. Zuletzt geprüft: 28 October 2008., EMEA: NutropinAq®: European Public Assessment Report (scientific discussion, 16.07.2008). Zuletzt geprüft: 28 October 2008. http://www.emea.europa.eu/humandocs/PDFs/EPAR/nutropinaq/437400en6.pdf
Rougeot C, Marchand P, Dray F, Girard F, Job JC, Pierson M, Ponte C, Rochiccioli P, Rappaport R: Comparative study of biosynthetic human growth hormone immunogenicity in growth hormone deficient children. Horm Res 1991, 35: 76–81. 10.1159/000181877
Zeisel HJ, Lutz A, von Petrykowski W: Immunogenicity of a mammalian cell-derived recombinant human growth hormone preparation during long-term treatment. Horm Res 1992, 37(Suppl 2):47–55. 10.1159/000182380
Acknowledgements
The authors thank from Samen Pharmaceutical Company to supply Samtropin and Norditropin rhGH for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
OT conceived of the study, collected and analyzed the data, drafted and edited the manuscript. MM conceived of the study, and participated in the design of the study. RH participated in the design of the study and performed the statistical analysis. ET participated in the design of the study. GSH helped to draft the manuscript. MR participated in the design of the study. AR participated in the design of the study. HA collected the data. SSH collected the data. FK coordinated the Lab. data. MQ performed the statistical analysis. SHR helped to draft the manuscript. BL conceived of the study and edited the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tabatabaei-Malazy, O., Mohajeri-Tehrani, M.R., Heshmat, R. et al. Efficacy and safety of Samtropin™ recombinant human growth hormone; a double-blind randomized clinical trial. J Diabetes Metab Disord 13, 115 (2014). https://doi.org/10.1186/s40200-014-0115-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40200-014-0115-0