Key Points
-
Antibody-based therapy for cancer has become established over the past 15 years and is now one of the most successful and important strategies for treating patients with haematological malignancies and solid tumours.
-
Evidence from clinical trials of antibodies in cancer patients has revealed the importance of iterative approaches for the selection of antigen targets and optimal antibodies.
-
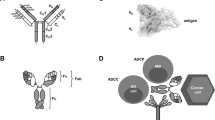
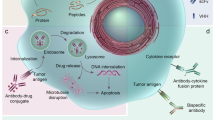
The killing of tumour cells using monoclonal antibodies (mAbs) can result from direct action of the antibody (through receptor blockade, for example), immune-mediated cell killing mechanisms, payload delivery, and specific effects of an antibody on the tumour vasculature and stroma.
-
Tumour antigens that have been successfully targeted include epidermal growth factor receptor (EGFR), ERBB2, vascular endothelial growth factor (VEGF), cytotoxic T lymphocyte-associated antigen 4 (CTLA4), CD20, CD30 and CD52.
-
Serological, genomic, proteomic and bioinformatic databases have also been used to identify antigens and receptors that are overexpressed in tumour cell populations or that are linked to gene mutations identified as driving cancer cell proliferation, including EGFRvIII, MET, CTLA4 and fibroblast activation protein (FAP).
-
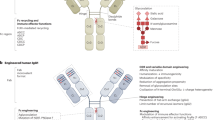
The successful development of candidate mAbs for the clinic involves a complex process of scientific and preclinical evaluations that include identification of the physical and chemical properties of the antibody; the detailed specificity analysis of antigen expression; the study of the immune effector functions and signalling pathway effects of the antibody; the analysis of in vivo antibody localization and distribution in transplanted or syngeneic tumour systems; and the observation of the in vivo therapeutic activity of the antibody.
-
A major objective for the clinical evaluation of mAbs has been determining the toxicity and therapeutic efficacy of the antibody alone or as a delivery system for radioisotopes or other toxic agents. It is also crucial to assess its in vivo specificity by determining its biodistribution in patients and to assess the ratio of antibody uptake in the tumour versus normal tissues.
-
Twelve antibodies have received approval from the US Food and Drug Administration for the treatment of various solid tumours and haematological malignancies, and a large number of additional therapeutic antibodies are currently being tested in early stage and late-stage clinical trials.
Abstract
The use of monoclonal antibodies (mAbs) for cancer therapy has achieved considerable success in recent years. Antibody–drug conjugates are powerful new treatment options for lymphomas and solid tumours, and immunomodulatory antibodies have also recently achieved remarkable clinical success. The development of therapeutic antibodies requires a deep understanding of cancer serology, protein-engineering techniques, mechanisms of action and resistance, and the interplay between the immune system and cancer cells. This Review outlines the fundamental strategies that are required to develop antibody therapies for cancer patients through iterative approaches to target and antibody selection, extending from preclinical studies to human trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rettig, W. J. & Old, L. J. Immunogenetics of human cell surface differentiation. Annu. Rev. Immunol. 7, 481–511 (1989).
Van den Eynde, B. J. & Scott, A. M. Encyclopedia of Immunology (eds Roitt, D. P. J. & Roitt, I. M.) 2424–2431 (Academic Press, London, 1998).
Scott, A. M. et al. A Phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptor. Proc. Natl Acad. Sci. USA 104, 4071–4076 (2007). An important trial describing the antibody targeting of a unique tumour-specific conformational receptor epitope in cancer patients and highlighting the relevance of early phase clinical trial design in antibody development.
Hughes, B. Antibody–drug conjugates for cancer: poised to deliver? Nature Rev. Drug Discov. 9, 665–667 (2010).
Weiner, L. M., Surana, R. & Wang, S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature Rev. Immunol. 10, 317–327 (2010).
Nelson, A. L. et al. Development trends for human monoclonal antibody therapeutics. Nature Rev. Drug Discov. 9, 767–774 (2010).
Deckert, P. M. Current constructs and targets in clinical development for antibody-based cancer therapy. Curr. Drug Targets 10, 158–175 (2009).
Ellis, L. M. & Hicklin, D. J. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nature Rev. Cancer. 8, 579–591 (2008).
Ravetch, J. In vivo veritas: the surprising roles of Fc receptors in immunity. Nature Immunol. 11, 183–185 (2010).
Schliemann, C. & Neri, D. Antibody-based vascular tumor targeting. Recent Results Cancer Res. 180, 201–216 (2010).
Scott, A. M. et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res. 9, 1639–1647 (2003).
Van Cutsem, E. et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 360, 1408–1417 (2009).
Hudis, C. A. Trastuzumab-mechanism of action and use in clinical practice. N. Engl. J. Med. 357, 39–51 (2007).
Weiner, G. J. Rituximab: mechanism of action. Semin. Hematol. 47, 115–123 (2010).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Eng. J. Med. 363, 711–723 (2010). A pivotal Phase III trial that led to the approval of ipilimumab for the treatment of patients with metastatic melanoma.
Chan, A. C. & Carter, P. J. Therapeutic antibodies for autoimmunity and inflammation. Nature Rev. Immunol. 10, 301–316 (2010).
Cheson, B. D. & Leonard, J. P. Monoclonal antibody therapy for B-cell non-Hodgkin's lymphoma. N. Engl. J. Med. 359, 613–626 (2008).
Schoeberl, B. et al. Therapeutically targeting ErbB3: a key node in ligand-induced activation of the ErbB receptor–PI3K axis. Sci. Signal. 2, ra31 (2009).
Canadas, I. et al. C-MET as a new therapeutic target for the development of novel anticancer drugs. Clin. Transl. Oncol. 12, 253–260 (2010).
Scartozzi, M. et al. Dalotuzumab, a recombinant humanized mAb targeted against IGFR1 for the treatment of cancer. Curr. Opin. Mol. Ther. 12, 361–371 (2010).
Vearing, C. et al. Concurrent binding of anti-EphA3 antibody and ephrin-A5 amplifies EphA3 signalling and downstream responses: potential as EphA3-specific tumour targeting reagents. Cancer Res. 65, 6745–6754 (2005).
Fox, N. L. et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor-1 and receptor-2 agonists for cancer therapy. Expert Opin. Biol. Ther. 10, 1–18 (2010).
Welt, S. et al. Antibody targeting in metastatic colon cancer: a phase I study of monoclonal antibody F19 against a cell surface protein of reactive tumor stromal fibroblasts. J. Clin. Oncol. 12, 1193–1203 (1994).
Jungbluth, A. A. et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc. Natl Acad. Sci. USA 100, 639–644 (2003).
Wu, A. M. & Senter, P. D. Arming antibodies: prospects and challenges for immunoconjugates. Nature Biotech. 23, 1137–1146 (2009).
Herbertson, R. A. et al. Phase I biodistribution and pharmacokinetic study of Lewis Y targeting immunoconjugate CMD-193 in patients with advanced epithelial cancers. Clin. Can. Res. 15, 6709–6715 (2009).
Caron, P. C. et al. A Phase IB trial of humanized monoclonal antibody M195 (anti-CD33) in myeloid leukemia: specific targeting without immunogenicity. Blood 83, 1760–1768 (1994).
Scott, A. M. et al. Specific targeting, biodistribution and lack of immunogenicity of chimeric anti-GD3 monoclonal antibody KM871 in patients with metastatic melanoma — results of a Phase I trial. J. Clin. Oncol. 19, 3976–3987 (2001).
Scott, A. M. et al. A Phase I biodistribution and pharmacokinetic trial of humanized monoclonal antibody hu3S193 in patients with advanced epithelial cancers which express the Lewis-y antigen. Clin. Cancer Res. 13, 3286–3292 (2007).
Divgi, C. R. et al. Phase I and imaging trial of indium 111-labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J. Natl Cancer Inst. 83, 97–104 (1991).
Dijkers, E. C. et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 87, 586–592 (2010).
de Bono, J. S. & Ashworth, A. Translating cancer research into targeted therapeutics. Nature 467, 543–549 (2010).
Strevel, E. L. & Siu, L. L. Cardiovascular toxicity of molecularly targeted agents. Eur. J. Cancer 45, 318–331 (2009).
Asnacios, A., Naveau, S. & Perlemuter, G. Gastrointestinal toxicities of novel agents in cancer therapy. Eur. J. Cancer 45, 332–342 (2009).
Friedman, H. S. et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 27, 4733–4740 (2009).
Amado, R. G. et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 1626–1634 (2008).
Karapetis, C. S. et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 359, 1757–1765 (2008). An important study demonstrating the lack of efficacy of wild-type EGFR-specific antibody cetuximab in patients with colorectal cancer harbouring KRAS mutations. This led to the approval of cetuximab only in patients with wild-type KRAS colorectal cancer.
Lievre, A. et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J. Clin. Oncol. 26, 374–379 (2008).
Leach, D. R., Krummel, M. F. & Allison J. P. Enhancement of antitumor immunity by CTLA-4. Science 271, 1734–1736 (1996). The first report of the enhancement of antitumour immunity following CTLA4 blockade.
Younes, A. et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 363, 1812–1821 (2010).
Lewis Phillips, G. D. et al. Targeting HER2-positive breast cancer with trastuzumab–DM1, an antibody–cytotoxic drug conjugate. Cancer Res. 68, 9280–9290 (2008).
Heiss, M. M. et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized Phase II/III trial. Int. J. Cancer 127, 2209–2221 (2010).
Boland, W. K. & Bebb, G. Nimotuzumab: a novel anti-EGFR monoclonal antibody that retains anti-EGFR activity while minimizing skin toxicity. Expert Opin. Biol. Ther. 9, 1199–1206 (2009).
Chen, S. et al. Pivotal study of iodine-131-labeled chimeric tumor necrosis treatment radioimmunotherapy in patients with advanced lung cancer. J. Clin. Oncol. 23, 1538–1547 (2005).
Brahmer, J. R. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics and immunologic correlates. J. Clin. Oncol. 28, 3167–3175 (2010).
Beatty, G. L. et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011).
Kirkwood, J. M. et al. Next generation of immunotherapy for melanoma. J. Clin. Oncol. 26, 3445–3455 (2008).
Taylor, C. et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin. Cancer Res. 15, 5133–5143 (2007).
Pillay, V., Gan, H. K. & Scott, A. M. Antibodies in oncology. N. Biotech. 28, 518–529 (2011).
Beck, A., Wurch, T., Bailly, C. & Corvaia, N. Strategies and challenges for the next generation of therapeutic antibodies. Nature Rev. Immunol. 10, 345–352 (2010).
Pillay, V. et al. The plasticity of oncogene addiction: implications for targeted therapies directed to receptor tyrosine kinases. Oncogene. 11, 448–458 (2009).
Weng, W. K. & Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 21, 3940–3947 (2003). This important study demonstrated the impact that FcγR polymorphisms have on the efficacy of rituximab in patients with follicular lymphoma, and highlights the importance of ADCC in the efficacy of rituximab.
Musolino, A. et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 26, 1789–1796 (2008).
Bibeau, F. et al. Impact of FcγRIIa–FcγRIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J. Clin. Oncol. 27, 1122–1129 (2009).
Ferris, R. L., Jaffee. E. M. & Ferrone, S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J. Clin. Oncol. 28, 4390–4399 (2010).
Acknowledgements
A.M.S. is supported by the Ludwig Institute for Cancer Research (LICR), National Health and Medical Research Council, Australia, grants 487922 and 1030469, and Operational Infrastructure Support funding from the Victorian government, Australia. J.D.W. is supported by LICR and the Cancer Research Institute (CRI), New York, USA. L.J.O. was supported by LICR and CRI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Fc function
-
The Fc portion of an antibody can activate a number of immunological pathways leading to tumour cell killing. This includes complement activation, activation of effector cells and phagocytosis of tumour cells.
- Pharmacokinetics
-
The process by which a drug is absorbed, distributed, metabolized and excreted from the body.
- Effector function
-
The biological effects of an antibody, which are usually immune-mediated and occur through Fc activation. This includes complement activation, and effector cell activation that leads to phagocytosis, opsonization or cytotoxic cell killing.
- Immunogenicity
-
The ability of a molecule (for example, an antibody) to induce an immune response in a human or other animal.
- Cytotoxic test
-
An assay that measures how toxic a compound is to cells.
- Alloantibodies
-
Antibodies that are produced following the immunization (with an alloantigen) of an individual of a species that lacks that particular antigen.
- Hybridoma technology
-
The process of producing hybrid cell lines by fusing an antibody-producing B cell with a myeloma cell that can grow in tissue culture, thus producing a hybridoma line that produces a monoclonal antibody of a single specificity.
- FcγRIIa-131H polymorphisms
-
An Fcγ receptor (FcγR) is a protein found on the surface of immune cells that binds the Fc of antibodies, and that facilitates the cytotoxic or phagocytic activity of these cells. Polymorphisms of FcγR genes may result in higher Fc binding in vitro and in vivo, with resulting enhanced cytotoxic activity of antibodies.
- Fucosylation modification
-
Engineered antibodies with core fucose residues removed from Fc N-glycans have increased binding to Fcγ receptor IIIa, resulting in enhanced antibody-dependent cellular cytotoxic activity.
Rights and permissions
About this article
Cite this article
Scott, A., Wolchok, J. & Old, L. Antibody therapy of cancer. Nat Rev Cancer 12, 278–287 (2012). https://doi.org/10.1038/nrc3236
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3236