Key Points
-
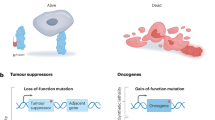
A primary goal of modern cancer drug development is the identification of targeted therapeutics that specifically kill tumour cells while leaving normal healthy cells unharmed.
-
Synthetic lethality began as a description of genetic interactions that were observed in model organisms, but it is rapidly growing into a major strategy in the search for the next generation of targeted cancer therapies.
-
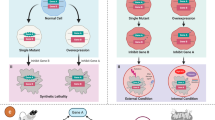
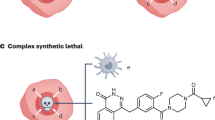
Synthetic lethality is based on the genetic interaction between two genes. Inhibition of either gene alone has no effect on viability, but the combined inhibition of the two genes results in cell death.
-
Cancer cells are frequently distinguished from normal cells by defects in specific genes that drive their growth and metastasis, and so the identification of genes or drugs that have a synthetic lethal interaction with cancer-promoting genes represents a compelling approach for the development of targeted therapies.
-
The advent of RNA interference technologies allows whole-genome screening to uncover novel genetic interactions, whereas screening of small-molecule libraries can reveal novel lead agents for cancers with specific genetic mutations. Mutations in both gain-of-function oncogenes and loss-of-function tumour suppressor genes can be targeted.
-
Conditional synthetic lethality exploits changes in gene expression that are induced by the tumour microenvironment, leading to an additional layer of specificity.
-
DNA repair genes that are mutated in some cancers have been the most common targets of synthetic lethality studies so far, and these studies are the most clinically developed. Among the findings are the selective sensitivity of BRCA-deficient breast cancer cells to poly(ADP-ribose) polymerase (PARP) inhibitors and the importance of certain DNA polymerases for the survival of colorectal carcinomas that are defective in DNA mismatch repair proteins. PARP inhibitors have demonstrated clinical efficacy in BRCA-mutant breast and ovarian tumours.
-
This Review explores how synthetic lethality screening can be used to identify new interactions and molecules for the treatment of cancer.
Abstract
Unique features of tumours that can be exploited by targeted therapies are a key focus of current cancer research. One such approach is known as synthetic lethality screening, which involves searching for genetic interactions of two mutations whereby the presence of either mutation alone has no effect on cell viability but the combination of the two mutations results in cell death. The presence of one of these mutations in cancer cells but not in normal cells can therefore create opportunities to selectively kill cancer cells by mimicking the effect of the second genetic mutation with targeted therapy. Here, we summarize strategies that can be used to identify synthetic lethal interactions for anticancer drug discovery, describe examples of such interactions that are currently being investigated in preclinical and clinical studies of targeted anticancer therapies, and discuss the challenges of realizing the full potential of such therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Druker, B. J. Perspectives on the development of a molecularly targeted agent. Cancer Cell 1, 31–36 (2002).
Hellman, S. & Vokes, E. E. Advancing current treatments for cancer. Sci. Am. 275, 118–123 (1996).
Oliff, A., Gibbs, J. B. & McCormick, F. New molecular targets for cancer therapy. Sci. Am. 275, 144–149 (1996).
Hynes, N. E. & Lane, H. A. ERBB receptors and cancer: the complexity of targeted inhibitors. Nature Rev. Cancer 5, 341–354 (2005).
Buchdunger, E. et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 56, 100–104 (1996).
Druker, B. J. et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 355, 2408–2417 (2006).
Druker, B. J. et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr–Abl positive cells. Nature Med. 2, 561–566 (1996). This preclinical study identified imatinib as a compound that was capable of interfering with tyrosine kinase activity of BCR–ABL in cell lines, primary tumour cells and in xenograft tumours.
Talpaz, M. et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 354, 2531–2541 (2006).
Druker, B. J. et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344, 1031–1037 (2001).
Pollack, V. A. et al. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J. Pharmacol. Exp. Ther. 291, 739–748 (1999).
Ansari, J., Palmer, D. H., Rea, D. W. & Hussain, S. A. Role of tyrosine kinase inhibitors in lung cancer. Anticancer Agents Med. Chem. 9, 569–575 (2009).
Mok, T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009).
Shepherd, F. A. et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 353, 123–132 (2005).
Ng, S. S., Tsao, M. S., Nicklee, T. & Hedley, D. W. Effects of the epidermal growth factor receptor inhibitor OSI-774, Tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinoma. Mol. Cancer Ther. 1, 777–783 (2002).
Moore, M. J. et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 25, 1960–1966 (2007).
Ahmad, T. & Eisen, T. Kinase inhibition with BAY 43-9006 in renal cell carcinoma. Clin. Cancer Res. 10, 6388S–6392S (2004).
Motzer, R. J. et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 24, 16–24 (2006).
Mendel, D. B. et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 9, 327–337 (2003).
Gu, J., Ruppen, M. E. & Cai, P. Lipase-catalyzed regioselective esterification of rapamycin: synthesis of temsirolimus (CCI-779). Org. Lett. 7, 3945–3948 (2005).
Sedrani, R., Cottens, S., Kallen, J. & Schuler, W. Chemical modification of rapamycin: the discovery of SDZ RAD. Transplant Proc. 30, 2192–2194 (1998).
Motzer, R. J. et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372, 449–456 (2008).
Hudes, G. et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 356, 2271–2281 (2007).
Escudier, B. et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370, 2103–2111 (2007).
Escudier, B. et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 356, 125–134 (2007).
Motzer, R. J. et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 356, 115–124 (2007).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Liu, L. et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 66, 11851–11858 (2006).
Force, T. & Kolaja, K. L. Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nature Rev. Drug Discov. 10, 111–126 (2011).
Janne, P. A., Gray, N. & Settleman, J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nature Rev. Drug Discov. 8, 709–723 (2009).
Hartwell, L. H., Szankasi, P., Roberts, C. J., Murray, A. W. & Friend, S. H. Integrating genetic approaches into the discovery of anticancer drugs. Science 278, 1064–1068 (1997). This paper proposed applying classical yeast genetics to discovering novel chemotherapies with a specific focus on DNA repair pathways.
Kroll, E. S., Hyland, K. M., Hieter, P. & Li, J. J. Establishing genetic interactions by a synthetic dosage lethality phenotype. Genetics 143, 95–102 (1996). This study describeda synthetic lethality screen in yeast to identify unknown interactions between different genes.
Kaelin, W. G. Jr. The concept of synthetic lethality in the context of anticancer therapy. Nature Rev. Cancer 5, 689–698 (2005).
Kaelin, W. G. Jr. Synthetic lethality: a framework for the development of wiser cancer therapeutics. Genome Med. 1, 99 (2009).
Bender, A. & Pringle, J. R. Use of a screen for synthetic lethal and multicopy suppressee mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae. Mol. Cell Biol. 11, 1295–1305 (1991).
Guarente, L. Synthetic enhancement in gene interaction: a genetic tool come of age. Trends Genet. 9, 362–366 (1993).
Hartman, J. L., Garvik, B. & Hartwell, L. Principles for the buffering of genetic variation. Science 291, 1001–1004 (2001).
Bryant, H. E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005). One of two papers identifying PARP inhibitors as synthetically lethalto BRCA mutations.
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005). The other paper identifying a genetic interaction between PARP inhibition (repair of single-stranded DNA breaks) and BRCA mutation (which was important for homologous recombinational repair of double-stranded DNA breaks).
Felsher, D. W. & Bishop, J. M. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell 4, 199–207 (1999).
Pelengaris, S., Littlewood, T., Khan, M., Elia, G. & Evan, G. Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol. Cell 3, 565–577 (1999).
Prochownik, E. V. & Vogt, P. K. Therapeutic targeting of Myc. Genes Cancer 1, 650–659 (2010).
Harris, C. C. & Hollstein, M. Clinical implications of the p53 tumor-suppressor gene. N. Engl. J. Med. 329, 1318–1327 (1993).
Olivier, M. et al. Recent advances in p53 research: an interdisciplinary perspective. Cancer Gene Ther. 16, 1–12 (2009).
Liu, T. C., Hwang, T. H., Bell, J. C. & Kirn, D. H. Translation of targeted oncolytic virotherapeutics from the lab into the clinic, and back again: a high-value iterative loop. Mol. Ther. 16, 1006–1008 (2008).
Gien, L. T. & Mackay, H. J. The emerging role of PARP inhibitors in the treatment of epithelial ovarian cancer. J. Oncol. 2010, 151750 (2010).
Tutt, A. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 376, 235–244 (2010). This paper demonstrated the use of PARP inhibitors for relapsed or metastatic breast cancer with BRCA mutations.
Audeh, M. W. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376, 245–251 (2010). This paper demonstrated the use of PARP inhibitors for relapsed or metastatic ovarian cancers with BRCA mutations.
Brummelkamp, T. R., Nijman, S. M., Dirac, A. M. & Bernards, R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature 424, 797–801 (2003). This paper describes one of the first large-scale RNAi library screens.
Ngo, V. N. et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature 441, 106–110 (2006).
Scholl, C. et al. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell 137, 821–834 (2009).
Luo, J. et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell 137, 835–848 (2009).
Einav, Y. et al. Replication and episomal maintenance of Epstein-Barr virus-based vectors in mouse embryonal fibroblasts enable synthetic lethality screens. Mol. Cancer Ther. 2, 1121–1128 (2003).
Simons, A., Dafni, N., Dotan, I., Oron, Y. & Canaani, D. Establishment of a chemical synthetic lethality screen in cultured human cells. Genome Res. 11, 266–273 (2001). This study used episomal gene transfer to circumvent genetic drift of isogenic cell lines.
Simons, A. H., Dafni, N., Dotan, I., Oron, Y. & Canaani, D. Genetic synthetic lethality screen at the single gene level in cultured human cells. Nucleic Acids Res. 29, e100 (2001).
Bindra, R. S. et al. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol. Cell Biol. 24, 8504–8518 (2004).
Chan, N. et al. Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res. 68, 605–614 (2008).
Chan, N. et al. Contextual synthetic lethality of cancer cell kill based on the tumor microenvironment. Cancer Res. 70, 8045–8054 (2010). This paper describesa conditional synthetic lethality whereby hypoxia suppresses DNA repair proteins and subsequently makes cells deficient in recombinational repair and, consequently, sensitive to PARP inhibition.
Chen, Y., Cairns, R., Papandreou, I., Koong, A. & Denko, N. C. Oxygen consumption can regulate the growth of tumors, a new perspective on the Warburg effect. PLoS ONE 4, e7033 (2009).
Michelakis, E. D., Webster, L. & Mackey, J. R. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br. J. Cancer 99, 989–994 (2008).
Banasik, M. & Ueda, K. Inhibitors and activators of ADP-ribosylation reactions. Mol. Cell. Biochem. 138, 185–197 (1994).
Loh, V. M. Jr et al. Phthalazinones. Part 1: the design and synthesis of a novel series of potent inhibitors of poly(ADP-ribose) polymerase. Bioorg. Med. Chem. Lett. 15, 2235–2238 (2005).
Pivazyan, A. D., Birks, E. M., Wood, T. G., Lin, T. S. & Prusoff, W. H. Inhibition of poly(ADP-ribose)polymerase activity by nucleoside analogs of thymidine. Biochem. Pharmacol. 44, 947–953 (1992).
Skalitzky, D. J. et al. Tricyclic benzimidazoles as potent poly(ADP-ribose) polymerase-1 inhibitors. J. Med. Chem. 46, 210–213 (2003).
Rouleau, M., Patel, A., Hendzel, M. J., Kaufmann, S. H. & Poirier, G. G. PARP inhibition: PARP1 and beyond. Nature Rev. Cancer 10, 293–301 (2010).
Hall, J. M. et al. Closing in on a breast cancer gene on chromosome 17q. Am. J. Hum. Genet. 50, 1235–1242 (1992).
Casey, G. et al. Functional evidence for a breast cancer growth suppressor gene on chromosome 17. Hum. Mol. Genet. 2, 1921–1927 (1993).
Wooster, R. et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12–13. Science 265, 2088–2090 (1994).
Parikh, B. & Advani, S. Pattern of second primary neoplasms following breast cancer. J. Surg. Oncol. 63, 179–182 (1996).
Petermann, E., Keil, C. & Oei, S. L. Importance of poly(ADP-ribose) polymerases in the regulation of DNA-dependent processes. Cell. Mol. Life Sci. 62, 731–738 (2005).
Ashworth, A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J. Clin. Oncol. 26, 3785–3790 (2008).
Fong, P. C. et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009).
Hutchinson, L. Targeted therapies: PARP inhibitor olaparib is safe and effective in patients with BRCA1 and BRCA2 mutations. Nature Rev. Clin. Oncol. 7, 549 (2010).
Turner, N., Tutt, A. & Ashworth, A. Hallmarks of 'BRCAness' in sporadic cancers. Nature Rev. Cancer 4, 814–819 (2004).
Efimova, E. V. et al. Poly(ADP-ribose) polymerase inhibitor induces accelerated senescence in irradiated breast cancer cells and tumors. Cancer Res. 70, 6277–6282 (2010).
Loser, D. A. et al. Sensitization to radiation and alkylating agents by inhibitors of poly(ADP-ribose) polymerase is enhanced in cells deficient in DNA double-strand break repair. Mol. Cancer Ther. 9, 1775–1787 (2010).
Bhattacharyya, A., Ear, U. S., Koller, B. H., Weichselbaum, R. R. & Bishop, D. K. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J. Biol. Chem. 275, 23899–23903 (2000).
Moynahan, M. E., Cui, T. Y. & Jasin, M. Homology-directed DNA repair, mitomycin-c resistance, and chromosome stability is restored with correction of a Brca1 mutation. Cancer Res. 61, 4842–4850 (2001).
Howlett, N. G. et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 297, 606–609 (2002).
Kraakman-van der Zwet, M. et al. Brca2 (XRCC11) deficiency results in radioresistant DNA synthesis and a higher frequency of spontaneous deletions. Mol. Cell Biol. 22, 669–679 (2002).
Evers, B. et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin. Cancer Res. 14, 3916–3925 (2008).
Rottenberg, S. et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl Acad. Sci. USA 105, 17079–17084 (2008).
Fong, P. C. et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J. Clin. Oncol. 28, 2512–2519 (2010).
Sakai, W. et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 451, 1116–1120 (2008).
Chan, S. L. & Mok, T. PARP inhibition in BRCA-mutated breast and ovarian cancers. Lancet 376, 211–213 (2010).
Gottipati, P. et al. Poly(ADP-ribose) polymerase is hyperactivated in homologous recombination-defective cells. Cancer Res. 70, 5389–5398 (2010).
Feng, Z. et al. Rad52 inactivation is synthetically lethal with BRCA2 deficiency. Proc. Natl Acad. Sci. USA 108, 686–691 (2011).
Willers, H. et al. Utility of DNA repair protein foci for the detection of putative BRCA1 pathway defects in breast cancer biopsies. Mol. Cancer Res. 7, 1304–1309 (2009).
Martin, S. A. et al. DNA polymerases as potential therapeutic targets for cancers deficient in the DNA mismatch repair proteins MSH2 or MLH1. Cancer Cell 17, 235–248 (2010).
Martin, S. A., Hewish, M., Sims, D., Lord, C. J. & Ashworth, A. Parallel high throughput RNA interference screens identify PINK1 as a potential therapeutic target for the treatment of DNA mismatch repair deficient cancers. Cancer Res. 71, 1836–1848 (2011).
Kinzler, K. W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Fishel, R. et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 75, 1027–1038 (1993).
Leach, F. S. et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 75, 1215–1225 (1993).
Strand, M., Prolla, T. A., Liskay, R. M. & Petes, T. D. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature 365, 274–276 (1993).
Koi, M. et al. Human chromosome 3 corrects mismatch repair deficiency and microsatellite instability and reduces N-methyl-N′-nitro-N-nitrosoguanidine tolerance in colon tumor cells with homozygous hMLH1 mutation. Cancer Res. 54, 4308–4312 (1994).
Campbell, J. L., Soll, L. & Richardson, C. C. Isolation and partial characterization of a mutant of Escherichia coli deficient in DNA polymerase II. Proc. Natl Acad. Sci. USA 69, 2090–2094 (1972).
Matsukage, A. et al. Assignment of the gene for human DNA polymerase β (POLB) to chromosome 8. Jpn J. Cancer Res. 77, 330–333 (1986).
Sobol, R. W. et al. Requirement of mammalian DNA polymerase-β in base-excision repair. Nature 379, 183–186 (1996).
Zullo, S. J. et al. Localization by fluorescence in situ hybridization (FISH) of human mitochondrial polymerase γ (POLG) to human chromosome band 15q24→q26, and of mouse mitochondrial polymerase γ (Polg) to mouse chromosome band 7E, with confirmation by direct sequence analysis of bacterial artificial chromosomes (BACs). Cytogenet. Cell Genet. 78, 281–284 (1997).
Ropp, P. A. & Copeland, W. C. Cloning and characterization of the human mitochondrial DNA polymerase, DNA polymerase γ. Genomics 36, 449–458 (1996).
Kaelin, W. G. Jr. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nature Rev. Cancer 8, 865–873 (2008).
Melmon, K. L. & Rosen, S. W. Lindau's disease. Review of the literature and study of a large kindred. Am. J. Med. 36, 595–617 (1964).
Gnarra, J. R. et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nature Genet. 7, 85–90 (1994).
Herman, J. G. et al. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc. Natl Acad. Sci. USA 91, 9700–9704 (1994).
Young, A. C. et al. Analysis of VHL gene alterations and their relationship to clinical parameters in sporadic conventional renal cell carcinoma. Clin. Cancer Res. 15, 7582–7592 (2009).
Ritchie, A. W. & Chisholm, G. D. The natural history of renal carcinoma. Semin. Oncol. 10, 390–400 (1983).
Motzer, R. J., Russo, P., Nanus, D. M. & Berg, W. J. Renal cell carcinoma. Curr. Probl. Cancer 21, 185–232 (1997).
Iliopoulos, O., Kibel, A., Gray, S. & Kaelin, W. G. Jr. Tumour suppression by the human von Hippel-Lindau gene product. Nature Med. 1, 822–826 (1995).
Bommi-Reddy, A. et al. Kinase requirements in human cells: III. Altered kinase requirements in VHL−/− cancer cells detected in a pilot synthetic lethal screen. Proc. Natl Acad. Sci. USA 105, 16484–16489 (2008).
Turcotte, S. et al. A molecule targeting VHL-deficient renal cell carcinoma that induces autophagy. Cancer Cell 14, 90–102 (2008).
Chan, D. A. & Giaccia, A. J. Targeting cancer cells by synthetic lethality: autophagy and VHL in cancer therapeutics. Cell Cycle 7, 2987–2990 (2008).
Turcotte, S. & Giaccia, A. J. Targeting cancer cells through autophagy for anticancer therapy. Curr. Opin. Cell Biol. 22, 246–251 (2010).
Varela, I. et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469, 539–542 (2011).
Neshat, M. S. et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc. Natl Acad. Sci. USA 98, 10314–10319 (2001).
Podsypanina, K. et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc. Natl Acad. Sci. USA 98, 10320–10325 (2001).
Thomas, G. V. et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nature Med. 12, 122–127 (2006). References 113–115 describe synthetic lethal interactions between mTOR inhibitors and mutation of the PTEN tumour suppressor protein.
Kau, T. R. et al. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell 4, 463–476 (2003).
Shen, W. H. et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128, 157–170 (2007).
Dedes, K. J. et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci. Transl. Med. 2, 53ra75 (2010).
McEllin, B. et al. PTEN loss compromises homologous recombination repair in astrocytes: implications for glioblastoma therapy with temozolomide or poly(ADP-ribose) polymerase inhibitors. Cancer Res. 70, 5457–5464 (2010).
Mendes-Pereira, A. M. et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol. Med. 1, 315–322 (2009).
Cho, K. R. & Vogelstein, B. Genetic alterations in the adenoma–carcinoma sequence. Cancer 70, 1727–1731 (1992).
Bommi-Reddy, A. & Kaelin, W. G. Jr. Slaying RAS with a synthetic lethal weapon. Cell Res. 20, 119–121 (2010).
Sawyers, C. L. Finding and drugging the vulnerabilities of RAS-dependent cancers. Cell 137, 796–798 (2009).
Torrance, C. J., Agrawal, V., Vogelstein, B. & Kinzler, K. W. Use of isogenic human cancer cells for high-throughput screening and drug discovery. Nature Biotech. 19, 940–945 (2001). This paper describes an isogenic cell synthetic lethality screen to target oncogenic RAS.
Sarthy, A. V. et al. Survivin depletion preferentially reduces the survival of activated K-Ras-transformed cells. Mol. Cancer Ther. 6, 269–276 (2007).
Puyol, M. et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 18, 63–73 (2010).
Malumbres, M., Pevarello, P., Barbacid, M. & Bischoff, J. R. CDK inhibitors in cancer therapy: what is next? Trends Pharmacol. Sci. 29, 16–21 (2008).
Gilad, O. et al. Combining ATR suppression with oncogenic Ras synergistically increases genomic instability, causing synthetic lethality or tumorigenesis in a dosage-dependent manner. Cancer Res. 70, 9693–9702 (2010).
Steegmaier, M. et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr. Biol. 17, 316–322 (2007).
Mujica, A. O., Hankeln, T. & Schmidt, E. R. A novel serine/threonine kinase gene, STK33, on human chromosome 11p15.3. Gene 280, 175–181 (2001).
Barbie, D. A. et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 462, 108–112 (2009).
Chien, Y. & White, M. A. Characterization of RalB-Sec5-TBK1 function in human oncogenesis. Methods Enzymol. 438, 321–329 (2008).
Meylan, E. et al. Requirement for NF-κB signalling in a mouse model of lung adenocarcinoma. Nature 462, 104–107 (2009).
Dolma, S., Lessnick, S. L., Hahn, W. C. & Stockwell, B. R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3, 285–296 (2003).
Yagoda, N. et al. RAS–RAF–MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447, 864–868 (2007).
Wang, Y. et al. Synthetic lethal targeting of MYC by activation of the DR5 death receptor pathway. Cancer Cell 5, 501–512 (2004).
Yang, D. et al. Therapeutic potential of a synthetic lethal interaction between the MYC proto-oncogene and inhibition of aurora-B kinase. Proc. Natl Acad. Sci. USA 107, 13836–13841 (2010).
Acknowledgements
This work was supported by the following grants from the US National Cancer Institute: NCI-CA-67166 (A.J.G), NCI-CA-88480 (A.J.G) and NCI-CA-123823 (D.A.C). It was also supported by a grant from Action to Cure Kidney Cancer (A.J.G.). We apologize to colleagues whose work we failed to cite.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Amato J. Giaccia is a founder of Ruga Corporation. Denise A. Chan is a scientific consultant for Ruga Corporation.
Related links
Related links
FURTHER INFORMATION
Glossary
- Small interfering RNA
-
(siRNA). A sequence of double-stranded RNA, generally 21 nucleotides in length, which targets specific mRNA sequences for degradation or inhibits translation of specific genes. Synthetic siRNAs can be introduced into a cell by transfection but they are short-lived.
- Poly(ADP-ribose) polymerase
-
(PARP). A family of enzymes that catalyses the conversion of nicotinamide adenine dinucleotide into nicotinamide and polymers of ADP-ribose at glutamic acid residues of nuclear proteins. These enzymes are involved in a variety of cellular processes, notably DNA repair.
- Short hairpin RNA
-
(shRNA). A plasmid or vector-based method for producing stable gene silencing. A promoter drives transcription of a target sequence, which forms a hairpin loop that is processed by the cellular RNA interference machinery, thereby forming small interfering RNAs to silence a particular gene.
- Replication fork
-
The structure formed from the unwinding and breaking of the hydrogen bond of the two strands of DNA during replication. Each individual strand of DNA becomes a template for replication.
- Knudson two-hit hypothesis
-
A model that proposes that cancer is a genetic disease and that successive genetic alterations in both alleles are needed to turn a normal cell into a cancer cell. In spontaneous cancers, two successive rare events must occur but in cases of hereditary susceptibility to cancer, inheritance of a damaged gene followed by a rare event results in mutation.
- Clonogenic survival curve
-
The standard method for determining the effectiveness of a particular treatment on the proliferation of cells. Cells are plated in a tissue culture dish and allowed to attach overnight. The plates are then treated and grown until single cells form colonies, which are then fixed, stained and counted.
- Autophagy
-
A catabolic process that sequesters and recycles cellular components, including organelles and long-lived proteins, in response to diverse stimuli. Autophagosomes form via invagination of the cell membrane, creating double-membrane vesicles. These autophagic vesicles then fuse with lysosomes, creating autophagolysosomes in which the contents of the cell are degraded by acidic lysosomal hydrolases.
Rights and permissions
About this article
Cite this article
Chan, D., Giaccia, A. Harnessing synthetic lethal interactions in anticancer drug discovery. Nat Rev Drug Discov 10, 351–364 (2011). https://doi.org/10.1038/nrd3374
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3374