Abstract
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease associated with aberrant activation of T and B lymphocytes. Abnormal activation of intracellular signaling molecules in lymphocytes by inflammatory cytokines can instigate the inflammation in SLE.
Materials and Methods
The activation of extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) in inflammatory cytokine IL-18-activated monocytes, CD4+ T helper (Th) lymphocytes, CD8+ T lymphocytes, and CD19+B lymphocytes in 22 SLE patients and 20 sex- and age-matched control subjects were measured by flow cytometry.
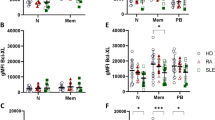
Results and Discussion
The basal expressions of phospho-p38 MAPK in CD4+ T lymphocytes, CD8+ T lymphocytes, and B lymphocytes were significantly higher in SLE patients than controls (all p < 0.05). The expression of phospho-p38 MAPK in CD4+ T lymphocytes, CD8+ T lymphocytes and B lymphocytes, and phospho-JNK in CD8+ T lymphocytes and B lymphocytes was also significantly elevated in SLE patients upon the activation by IL-18, exhibiting significant correlation with the plasma concentrations of Th1 chemokine CXCL10 (all p < 0.05). The expression of phospho-JNK in IL-18 activated CD8+ T lymphocytes and the relative % fold increase of the expression of phospho-JNK upon IL-18 activation in B lymphocytes were significantly correlated with SLE disease activity index (both p < 0.05).
Conclusion
The inflammation-mediated activation of JNK and p38 MAPK signaling pathways in T and B lymphocytes can be the underlying intracellular mechanisms causing lymphocyte hyperactivity in SLE.




Similar content being viewed by others
References
La Cava A. Lupus and T cells. Lupus. 2009;18:196–201.
Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40.
Wong CK, Ip WK, Lam CWK. Biochemical assessment of intracellular signal transduction pathways in eosinophils: implications for pharmacotherapy. Critical Rev Clin Lab Sci. 2004;41:79–113.
de Groot RP, Coffer PJ, Koenderman L. Regulation of proliferation, differentiation and survival by the IL-3/IL-5/GM-CSF receptor family. Cell Signal. 1998;10:619–28.
Raingeaud J, Gupta S, Rogers JS, et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen- activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–6.
Derijard B, Hibi M, Wu IH, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–37.
Wong CK, Cheung PF, Ip WK, Lam CWK. IL-25 induced chemokines and IL-6 release from eosinophils is mediated by p38 MAPK, JNK and NF-κB. Am J Respir Cell Mol Biol. 2005;33:186–94.
Cheung PFY, Wong CK, Ip WK, Lam CWK. IL-25 regulates adhesion molecule expression on eosinophils: mechanism of eosinophilia in allergic inflammation. Allergy. 2006;61:878–85.
Wong CK, Li PW, Lam CW. Intracellular JNK, p38 MAPK and NF-kappaB regulate IL-25 induced release of cytokines and chemokines from costimulated T helper lymphocytes. Immunol Lett. 2007;112:82–91.
Wong CK, Lit LCW, Li EK, Tam LS, Lam CWK. Aberrant production of soluble costimulatory molecules CTLA-4, CD28, CD80 and CD86 in patients with systemic lupus erythematosus. Rheumatology. 2005;44:989–94.
Wong HK, Kammer GM, Dennis G, Tsokos GC. Abnormal NF-kappa B activity in T lymphocytes from patients with systemic lupus erythematosus is associated with decreased p65-RelA protein expression. J Immunol. 1999;163:1682–9.
Gorjestani S, Rider V, Kimler BF, Greenwell C, Abdou NI. Extracellular signal-regulated kinase 1/2 signalling in SLE T cells is influenced by oestrogen and disease activity. Lupus. 2008;17:548–54.
Liossis SN, Solomou EE, Dimopoulos MA, Panayiotidis P, Mavrikakis MM, Sfikakis PP. B-cell kinase lyn deficiency in patients with systemic lupus erythematosus. J Investig Med. 2001;49:157–65.
Jury EC, Kabouridis PS, Abba A, Mageed RA, Isenberg DA. Increased ubiquitination and reduced expression of LCK in T lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1343–54.
Deng C, Kaplan MJ, Yang J, et al. Decreased Ras-mitogen-activated protein kinase signaling may cause DNA hypomethylation in T lymphocytes from lupus patients. Arthritis Rheum. 2001;44:397–407.
Dinarello CA. IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24.
Hoshino T, Wiltrout RH, Young HA. IL-18 is a potent coinducer of IL-13 in NK and T cells: a new potential role for IL-18 in modulating the immune response. J Immunol. 1999;162:5070–77.
Yoshimoto T, Mizutani H, Tsutsui H, et al. IL-18 induction of IgE: dependence on CD4+ T cells, IL-4 and STAT6. Nat Immunol. 2000;1:132–7.
Wong CK, Li EK, Ho CY, Lam CWK. Elevation of plasma interleukin-18 concentration is correlated with disease activity in systemic lupus erythematosus. Rheumatol. 2000;39:1078–81.
Wong CK, Ho CY, Li EK, Tam LS, Lam CW. Elevated production of interleukin-18 is associated with renal disease in patients with systemic lupus erythematosus. Clin Exp Immunol. 2002;130:345–51.
Wong CK, Lit LCW, Tam LS, Li EK, Wong PTY, Lam CWK. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–93.
Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7.
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH, the Committee on Prognosis studies in SLE. Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–40.
Grammer AC, Fischer R, Lee O, Zhang X, Lipsky PE. Flow cytometric assessment of the signaling status of human B lymphocytes from normal and autoimmune individuals. Arthritis Res Ther. 2004;6:28–38.
Lit LC, Wong CK, Tam LS, Li EK, Lam CW. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann Rheum Dis. 2006;65:209–15.
Pascual V, Banchereau J, Palucka AK. The central role of dendritic cells and interferon-alpha in SLE. Curr Opin Rheumatol. 2003;15:548–56.
Cedeño S, Cifarelli DF, Blasini AM, et al. Defective activity of ERK-1 and ERK-2 mitogen-activated protein kinases in peripheral blood T lymphocytes from patients with systemic lupus erythematosus: potential role of altered coupling of Ras guanine nucleotide exchange factor hSos to adapter protein Grb2 in lupus T cells. Clin Immunol. 2003;106:41–9.
Thiel MJ, Schaefer CJ, Lesch ME, et al. Central role of the MEK/ERK MAP kinase pathway in a mouse model of rheumatoid arthritis: potential proinflammatory mechanisms. Arthritis Rheum. 2007;56:3347–57.
Sweeney SE, Firestein GS. Signal transduction in rheumatoid arthritis. Curr Opin Rheumatol. 2004;16:231–7.
Wang XS, Diener K, Manthey CL, et al. Molecular cloning and characterization of a novel p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:23668–74.
Rovin GH, Wilmer WA, Danne M, Dickerso JA, Dixon CL, Lu L. The mitogen-activated protein kinase p38 is necessary for interleukin 1-induced monocyte chemoattractant protein 1 expression by human mesangial cells. Cytokine. 1999;11:118–26.
Goebeler M, Kilian K, Gillizer R, et al. The MKK6/p38 stress kinase cascade is critical for tumor necrosis factor- -induced expression of monocyte-chemoattractant protein-1 in endothelial cells. Blood. 1999;93:857–65.
Deng C, Lu Q, Zhang Z, et al. Hydralazine may induce autoimmunity by inhibiting extracellular signal-regulated kinase pathway signaling. Arthritis Rheum. 2003;48:746–56.
Iwata Y, Wada T, Furuichi K, et al. p38 Mitogen-activated protein kinase contributes to autoimmune renal injury in MRL-Fas lpr mice. J Am Soc Nephrol. 2003;14:57–67.
Bishop GA, Hsing Y, Hostiger BS, et al. Molecular mechanism of B lymphocyte activation by the immune response modifier R-848. J Immunol. 2000;165:5552–7.
Yoshimoto K, Takahashi Y, Ogasawara M, et al. Aberrant expression of BAFF in T cells of systemic lupus erythematosus, which is recapitulated by a human T cell line, Loucy. Int Immunol. 2006;18:1189–96.
Peng SL, Gerth AJ, Ranger AM, Glimcher LH. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity. 2001;14:13–20.
Pessler F, Dai L, Cron RQ, Schumacher HR. NFAT transcription factors—new players in the pathogenesis of inflammatory arthropathies? Autoimmun Rev. 2006;5:106–10.
O’Neill LA. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov. 2006;5:549–63.
Park HJ, Lee HJ, Choi MS, et al. JNK pathway is involved in the inhibition of inflammatory target gene expression and NF-kappaB activation by melittin. J Inflamm (London). 2008;5:7.
Acknowledgment
The study was supported by a Direct Grant for Research (2041272), The Chinese University of Hong Kong.
Conflict of interest
No conflict of interest has been declared by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, C.K., Wong, P.T.Y., Tam, L.S. et al. Activation Profile of Intracellular Mitogen-Activated Protein Kinases in Peripheral Lymphocytes of Patients with Systemic Lupus Erythematosus. J Clin Immunol 29, 738–746 (2009). https://doi.org/10.1007/s10875-009-9318-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-009-9318-4