Key Points
-
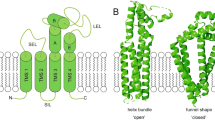
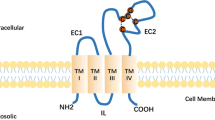
There are 33 mammalian tetraspanin proteins, each with characteristic structural features, including a conserved CCG motif in the large extracellular loop. Genetic evidence in fungi, worms, flies, mice and humans establishes that tetraspanins have key roles in many processes including development, fertilization, invasion and immune-cell function. Tetraspanins, which are expressed on nearly all cell and tissue types, also modulate cell morphology, motility, invasion, fusion, adhesion strengthening, signalling and protein trafficking.
-
Tetraspanins organize laterally, into tetraspanin-enriched microdomains (TEMs). At the core of TEMs are tetraspanins engaging in direct protein–protein interactions with themselves and other proteins, including the immunoglobulin superfamily members EWI-2 and EWI-F, Claudin-1, epidermal growth factor receptor (EGFR) membrane-bound ligands, integrins and Syntenin-1. These primary complexes are then joined into a network of looser secondary interactions involving many additional proteins. Tetraspanins and many of their partner proteins (for example, integrins, EWI proteins and Claudin-1) undergo protein palmitoylation, which helps to stabilize secondary interactions within TEMs.
-
Tetraspanins contribute to a number of normal and pathological processes that could be targeted therapeutically. For example, CD151 may support primary tumour growth as well as metastasis and angiogenesis, whereas tetraspanins CD9 and CD81 are required for oocyte fertilization. In addition, several tetraspanins contribute to the functions of platelets and lymphocytes, thereby enhancing blood clotting and affecting numerous immune functions.
-
Tetraspanins make substantial contributions towards infectious-disease pathologies. For HIV-1, human T-cell lymphotropic virus type 1 and other viruses, tetraspanins affect virus-induced cell fusion events and/or virus assembly and release. In hepatocytes, tetraspanin CD81 is needed for the initial steps in hepatitis C virus binding and infection, and for invasion by sporozoites from malaria-causing parasites.
-
Promising in vivo results suggest that targeting of tetraspanins may be therapeutically useful for injury repair, for cancer models and for combating infectious diseases. Anti-tetraspanin monoclonal antibodies, tetraspanin-derived recombinant soluble extracellular loops and RNAi knockdown strategies have all shown potential for effective modulation of tetraspanin functions.
Abstract
The tetraspanin transmembrane proteins have emerged as key players in malignancy, the immune system, during fertilization and infectious disease processes. Tetraspanins engage in a wide range of specific molecular interactions, occurring through the formation of tetraspanin-enriched microdomains (TEMs). TEMs therefore serve as a starting point for understanding how tetraspanins affect cell signalling, adhesion, morphology, motility, fusion and virus infection. An abundance of recent evidence suggests that targeting tetraspanins, for example, by monoclonal antibodies, soluble large-loop proteins or RNAi technology, should be therapeutically beneficial.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Huang, S. et al. The phylogenetic analysis of tetraspanins projects the evolution of cell–cell interactions from unicellular to multicellular organisms. Genomics 86, 674–684 (2005).
Garcia-Espana, A. et al. Appearance of new tetraspanin genes during vertebrate evolution. Genomics 91, 326–334 (2008).
Stipp, C. S., Kolesnikova, T. V. & Hemler, M. E. Functional domains in tetraspanin proteins. Trends Biochem. Sci. 28, 106–112 (2003).
Seigneuret, M., Delaguillaumie, A., Lagaudriere-Gesbert, C. & Conjeaud, H. Structure of the tetraspanin main extracellular domain. A partially conserved fold with a structurally variable domain insertion. J. Biol. Chem. 276, 40055–40064 (2001).
Boucheix, C. & Rubinstein, E. Tetraspanins. Cell. Mol. Life Sci. 58, 1189–1205 (2001).
Maecker, H. T., Todd, S. C. & Levy, S. The tetraspanin superfamily: molecular facilitators. FASEB J. 11, 428–442 (1997).
Kitadokoro, K. et al. CD81 extracellular domain 3D structure: insight into the tetraspanin superfamily structural motifs. EMBO J. 20, 12–18 (2001). The first detailed structural information for a tetraspanin LEL.
Min., G., Wang, H., Sun, T. T. & Kong, X. P. Structural basis for tetraspanin functions as revealed by the cryo-EM structure of uroplakin complexes at 6-Å resolution. J. Cell Biol. 173, 975–983 (2006). The most detailed structural information yet available for a tetraspanin and its partner protein.
Seigneuret, M. Complete predicted three-dimensional structure of the facilitator transmembrane protein and hepatitis C virus receptor CD81: conserved and variable structural domains in the tetraspanin superfamily. Biophys. J. 90, 212–227 (2006).
Kovalenko, O. V., Metcalf, D. G., DeGrado, W. F. & Hemler, M. E. Structural organization and interactions of transmembrane domains in tetraspanin proteins. BMC Struct. Biol. 5, 11 (2005).
Kazarov, A. R., Yang, X., Stipp, C. S., Sehgal, B. & Hemler, M. E. An extracellular site on tetraspanin CD151 determines α3 and α6 integrin-dependent cellular morphology. J. Cell Biol. 158, 1299–1309 (2002).
Berditchevski, et al. Analysis of the CD151-α3β1 integrin and CD151-tetraspanin interactions by mutagenesis. J. Biol. Chem. 276, 41165–41174 (2001).
Charrin, S. et al. EWI-2 is a new component of the tetraspanin web in hepatocytes and lymphoid cells. Biochem. J. 373, 409–421 (2003).
Kovalenko, O. V., Yang, X. H. & Hemler, M. E. A novel cysteine cross-linking method reveals a direct association between claudin-1 and tetraspanin CD9. Mol. Cell Proteomics 6, 1855–1867 (2007).
Wright, M. D., Moseley, G. W. & van Spriel, A. B. Tetraspanin microdomains in immune cell signalling and malignant disease. Tissue Antigens 64, 533–542 (2004).
Kishimoto, T. et al. Leukocyte Typing VI 1–1342 (Garland, New York, 1998).
Berditchevski, F. & Odintsova, E. Tetraspanins as regulators of protein trafficking. Traffic 8, 89–96 (2007).
Levy, S. & Shoham, T. The tetraspanin web modulates immune-signalling complexes. Nature Rev. Immunol. 5, 136–148 (2005).
Hemler, M. E. Tetraspanin proteins mediate cellular penetration, invasion and fusion events, and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 19, 397–422 (2003).
Rubinstein, E., Ziyyat, A., Wolf, J. P., Le Naour, F. & Boucheix, C. The molecular players of sperm–egg fusion in mammals. Semin. Cell Dev. Biol. 17, 254–263 (2006).
Lambou, K. et al. Fungi have three tetraspanin families with distinct functions. BMC Genomics 9, 63 (2008).
Todres, E., Nardi, J. B. & Robertson, H. M. The tetraspanin superfamily in insects. Insect Mol. Biol. 9, 581–590 (2000).
Hemler, M. E. Tetraspanin functions and associated microdomains. Nature Rev. Mol. Cell Biol. 6, 801–811 (2005).
Pileri, P. et al. Binding of hepatitis C virus to CD81. Science 282, 938–941 (1998). First evidence for HCV binding to tetraspanin CD81.
Wu, X. R., Sun, T. T. & Medina, J. J. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc. Natl Acad. Sci. USA 93, 9630–9635 (1996).
Ellerman, D. A., Ha, C., Primakoff, P., Myles, D. G. & Dveksler, G. S. Direct binding of the ligand PSG17 to CD9 requires a CD9 site essential for sperm–egg fusion. Mol. Biol. Cell 14, 5098–5103 (2003).
Odintsova, E. et al. Gangliosides play an important role in the organisation of CD82-enriched microdomains. Biochem. J. 400, 315–325 (2006).
Nydegger, S., Khurana, S., Krementsov, D. N., Foti, M. & Thali, M. Mapping of tetraspanin-enriched microdomains that can function as gateways for HIV-1. J. Cell Biol. 173, 795–807 (2006). An excellent characterization of TEMs on the surface of intact cells.
Ono, M. et al. GM3 ganglioside inhibits CD9-facilitated haptotactic cell motility: coexpression of GM3 and CD9 is essential in the downregulation of tumor cell motility and malignancy. Biochemistry 40, 6414–6421 (2001).
Charrin, S. et al. A physical and functional link between cholesterol and tetraspanins. Eur. J. Immunol. 33, 2479–2489 (2003). Definitive crosslinking evidence for cholesterol being a component of TEMs.
Claas, C., Stipp, C. S. & Hemler, M. E. Evaluation of prototype TM4SF protein complexes and their relation to lipid rafts. J. Biol. Chem. 276, 7974–7984 (2001). First paper to compare and distinguish TEMs and lipid rafts.
Yang, X. et al. Palmitoylation of tetraspanin proteins: modulation of CD151 lateral interactions, subcellular distribution, and integrin-dependent cell morphology. Mol. Biol. Cell 13, 767–781 (2002).
Berditchevski, F., Odintsova, E., Sawada, S. & Gilbert, E. Expression of the palmitoylation-deficient CD151 weakens the association of α3β1 integrin with the tetraspanin-enriched microdomains and affects integrin-dependent signalling. J. Biol. Chem. 277, 36991–37000 (2002).
Charrin, S. et al. Differential stability of tetraspanin/tetraspanin interactions: role of palmitoylation. FEBS Lett. 516, 139–144 (2002).
Kropshofer, H. et al. Tetraspan microdomains distinct from lipid rafts enrich select peptide-MHC class II complexes. Nature Immunol. 3, 61–68 (2002).
Le Naour, F., Andre, M., Boucheix, C. & Rubinstein, E. Membrane microdomains and proteomics: lessons from tetraspanin microdomains and comparison with lipid rafts. Proteomics 6, 6447–6454 (2006).
Kovalenko, O. V., Yang, X., Kolesnikova, T. V. & Hemler, M. E. Evidence for specific tetraspanin homodimers: inhibition of palmitoylation makes cysteine residues available for cross-linking. Biochem. J. 377, 407–417 (2004).
Tarrant, J. M., Robb, L., van Spriel, A. B. & Wright, M. D. Tetraspanins: molecular organisers of the leukocyte surface. Trends Immunol. 24, 610–617 (2003).
Zhang, X. A., Bontrager, A. L. & Hemler, M. E. TM4SF proteins associate with activated PKC and Link PKC to specific β1 integrins. J. Biol. Chem. 276, 25005–25013 (2001).
Chattopadhyay, N., Wang, Z., Ashman, L. K., Brady-Kalnay, S. M. & Kreidberg, J. A. α3β1 integrin-CD151, a component of the cadherin-catenin complex, regulates PTPmu expression and cell-cell adhesion. J. Cell Biol. 163, 1351–1362 (2003).
Berditchevski, F., Tolias, K. F., Wong, K., Carpenter, C. L. & Hemler, M. E. A novel link between integrins, TM4SF proteins (CD63, CD81) and phosphatidylinositol 4-kinase. J. Biol. Chem. 272, 2595–2598 (1997).
Yauch, R. L. & Hemler, M. E. Specific interactions among transmembrane 4 superfamily (TM4SF) proteins and phosphatidylinositol 4-kinase. Biochem. J. 351, 629–637 (2000).
Yang, X. et al. Palmitoylation supports assembly and function of integrin–tetraspanin complexes. J. Cell Biol. 167, 1231–1240 (2004).
Takeda, Y. et al. Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood 109, 1524–1532 (2007). First in vivo evidence for a tetraspanin affecting angiogenesis.
Yang, X. H. et al. CD151 accelerates breast cancer by regulating a6 integrin functions, signaling, and molecular organization. Cancer Res. 68, 3204–3213 (2008). First in vivo evidence for CD151 accelerating progression of breast cancer.
Silvie, O. et al. Cholesterol contributes to the organization of tetraspanin-enriched microdomains and to CD81-dependent infection by malaria sporozoites. J. Cell Sci. 119, 1992–2002 (2006). Use of a novel anti-CD81 mAb to demonstrate a coordinated role for cholesterol and CD81, on hepatocytes, during malaria infection.
Escola, J. M. et al. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B- lymphocytes. J. Biol. Chem. 273, 20121–20127 (1998).
Gesierich, S., Berezovskiy, I., Ryschich, E. & Zoller, M. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res. 66, 7083–7094 (2006).
Ang, J., Lijovic, M., Ashman, L. K., Kan, K. & Frauman, A. G. CD151 protein expression predicts the clinical outcome of low-grade primary prostate cancer better than histologic grading: a new prognostic indicator? Cancer Epidemiol. Biomarkers Prev. 13, 1717–1721 (2004).
Tokuhara, T. et al. Clinical significance of CD151 gene expression in non-small cell lung cancer. Clin. Cancer Res. 7, 4109–4114 (2001).
Kohno, M., Hasegawa, H., Miyake, M., Yamamoto, T. & Fujita, S. CD151 enhances cell motility and metastasis of cancer cells in the presence of focal adhesion kinase. Int. J. Cancer 97, 336–343 (2002).
Testa, J. E., Brooks, P. C., Lin, J. M. & Quigley, J. P. Eukaryotic expression cloning with an antimetastatic monoclonal antibody identifies a tetraspanin (PETA-3/CD151) as an effector of human tumor cell migration and metastasis. Cancer Res. 59, 3812–3820 (1999).
Zijlstra, A., Lewis, J., Degryse, B., Stuhlmann, H. & Quigley, J. P. The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell 13, 221–234 (2008).
Kreidberg, J. A. et al. α3β1 integrin has a crucial role in kidney and lung organogenesis. Development 122, 3537–3547 (1996).
Georges-Labouesse, E. N. et al. Absence of the α-6 integrin leads to epidermolysis bullosa and neonatal death in mice. Nature Genet. 13, 370–373 (1996).
Feltri, M. L., Arona, M., Scherer, S. S. & Wrabetz, L. Cloning and sequence of the cDNA encoding the β4 integrin subunit in rat peripheral nerve. Gene 186, 299–304 (1997).
Wright, M. D. et al. Characterization of mice lacking the tetraspanin superfamily member CD151. Mol. Cell Biol. 24, 5978–5988 (2004).
Sachs, N. et al. Kidney failure in mice lacking the tetraspanin CD151. J. Cell Biol. 175, 33–39 (2006).
Karamatic Crew, V. et al. CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood 104, 2217–2223 (2004).
Herlevsen, M., Schmidt, D. S., Miyazaki, K. & Zoller, M. The association of the tetraspanin D6.1A with the α6β4 integrin supports cell motility and liver metastasis formation. J. Cell Sci. 116, 4373–4390 (2003).
Claas, C. et al. Association between rat homologue of CO-029, a metastasis-associated tetraspanin molecule and consumption coagulopathy. J. Cell Biol. 141, 267–280 (1998).
Kanetaka, K. et al. Overexpression of tetraspanin CO-029 in hepatocellular carcinoma. J. Hepatol. 35, 637–642 (2001).
Zhou, Z. et al. TM4SF3 promotes esophageal carcinoma metastasis via upregulating ADAM12m expression. Clin. Exp.Metastasis 25, 537–548 (2008).
Ikeyama, S., Koyama, M., Yamaoko, M., Sasada, R. & Miyake, M. Suppression of cell motility and metastasis by transfection with human motility-related protein (MRP-1/CD9) DNA. J. Exp. Med. 177, 1231–1237 (1993).
Takeda, T. et al. Adenoviral transduction of MRP-1/CD9 and KAI1/CD82 inhibits lymph node metastasis in orthotopic lung cancer model. Cancer Res. 67, 1744–1749 (2007). In vivo demonstration of potential therapeutic use of tetraspanin tumour suppressor activity.
Saito, Y. et al. Absence of CD9 enhances adhesion-dependent morphologic differentiation, survival, and matrix metalloproteinase-2 production in small cell lung cancer cells. Cancer Res. 66, 9557–9565 (2006).
Ovalle, S. et al. The tetraspanin CD9 inhibits the proliferation and tumorigenicity of human colon carcinoma cells. Int. J. Cancer 121, 2140–2152 (2007).
Huang, H., Sossey-Alaoui, K., Beachy, S. H. & Geradts, J. The tetraspanin superfamily member NET-6 is a new tumor suppressor gene. J. Cancer Res. Clin. Oncol. 133, 761–769 (2007).
Radford, K. J., Mallesch, J. & Hersey, P. Suppression of human melanoma cell growth and metastasis by the melanoma-associated antigen CD63 (ME491). Int. J. Cancer 62, 631–635 (1995).
Moseley, G. W., Elliott, J., Wright, M. D., Partridge, L. J. & Monk, P. N. Interspecies contamination of the KM3 cell line: implications for CD63 function in melanoma metastasis. Int. J. Cancer 105, 613–616 (2003).
Jang, H. I. & Lee, H. A decrease in the expression of CD63 tetraspanin protein elevates invasive potential of human melanoma cells. Exp. Mol. Med. 35, 317–323 (2003).
Liu, W. M. & Zhang, X. A. KAI1/CD82, a tumor metastasis suppressor. Cancer Lett. 240, 183–194 (2006).
Dong, J.-T. et al. KAI 1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science 268, 884–886 (1995).
Odintsova, E., Voortman, J., Gilbert, E. & Berditchevski, F. Tetraspanin CD82 regulates compartmentalisation and ligand-induced dimerization of EGFR. J. Cell Sci. 116, 4557–4566 (2003).
Bass, R. et al. Regulation of urokinase receptor proteolytic function by the tetraspanin CD82. J. Biol. Chem. 280, 14811–14818 (2005).
He, B. et al. Tetraspanin CD82 attenuates cellular morphogenesis through down-regulating integrin a6-mediated cell adhesion. J. Biol. Chem. 280, 3346–3354 (2004).
Zheng, Z. Z. & Liu, Z. X. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates CD151-induced endothelial cell proliferation and cell migration. Int. J. Biochem. Cell Biol. 39, 340–348 (2006).
Dorrell, M. I., Aguilar, E. & Friedlander, M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest. Ophthalmol. Vis. Sci. 43, 3500–3510 (2002).
Rubinstein, E. et al. Reduced fertility of female mice lacking CD81. Dev. Biol. 290, 351–358 (2006).
Runge, K. E. et al. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev. Biol. 304, 317–325 (2007).
Stipp, C. S., Kolesnikova, T. V. & Hemler, M. E. EWI-2 regulates α3β1 integrin-dependent cell functions on laminin-5. J. Cell Biol. 163, 1167–1177 (2003).
Sala-Valdes, M. et al. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J. Biol. Chem. 281, 19665–19675 (2006).
Ziyyat, A. et al. CD9 controls the formation of clusters that contain tetraspanins and the integrin α6β1, which are involved in human and mouse gamete fusion. J. Cell Sci. 119, 416–424 (2006).
Primakoff, P. & Myles, D. G. Cell–cell membrane fusion during mammalian fertilization. FEBS Lett. 581, 2174–2180 (2007).
Miyazaki, T., Muller, U. & Campbell, K. S. Normal development but differentially altered proliferative responses of lymphocytes in mice lacking CD81. EMBO J. 16, 4217–4225 (1997).
Knobeloch, K. P. et al. Targeted inactivation of the tetraspanin CD37 impairs T-cell-dependent B-cell response under suboptimal costimulatory conditions. Mol. Cell Biol. 20, 5363–5369 (2000).
Tarrant, J. M. et al. The absence of Tssc6, a member of the tetraspanin superfamily, does not affect lymphoid development but enhances in vitro T-cell proliferative responses. Mol. Cell Biol. 22, 5006–5018 (2002).
van Spriel, A. B. et al. A regulatory role for CD37 in T cell proliferation. J. Immunol. 172, 2953–2961 (2004).
Shoham, T., Rajapaksa, R., Kuo, C. C., Haimovich, J. & Levy, S. Building of the tetraspanin web: distinct structural domains of CD81 function in different cellular compartments. Mol. Cell Biol. 26, 1373–1385 (2006). Comprehensive domain-swapping reveals distinct functions for different domains within CD81.
Feigelson, S. W., Grabovsky, V., Shamri, R., Levy, S. & Alon, R. The CD81 tetraspanin facilitates instantaneous leukocyte VLA-4 adhesion strengthening to vascular cell adhesion molecule 1 (VCAM-1) under shear flow. J. Biol. Chem. 278, 51203–51212 (2003). Strongly supports the concept of tetraspanins as modulators of integrin-dependent adhesion strengthening, rather than initial ligand binding.
Ha, S. A. et al. Regulation of B1 cell migration by signals through Toll-like receptors. J. Exp. Med. 203, 2541–2550 (2006).
Mittelbrunn, M., Yanez-Mo, M., Sancho, D., Ursa, A. & Sanchez-Madrid, F. Cutting Edge: dynamic redistribution of tetraspanin CD81 at the central zone of the immune synapse in both T lymphocytes and APC. J. Immunol. 169, 6691–6695 (2002).
Mazurov, D., Heidecker, G. & Derse, D. HTLV-1 Gag protein associates with CD82 tetraspanin microdomains at the plasma membrane. Virology 346, 194–204 (2006).
Unternaehrer, J. J., Chow, A., Pypaert, M., Inaba, K. & Mellman, I. The tetraspanin CD9 mediates lateral association of MHC class II molecules on the dendritic cell surface. Proc. Natl Acad. Sci. USA 104, 234–239 (2007).
Lau, L. M. et al. The tetraspanin superfamily member, CD151 regulates outside-in integrin αIIbβ3 signalling and platelet function. Blood 104, 2368–2375 (2004).
Goschnick, M. W. et al. Impaired “outside-in” integrin αIIbβ3 signaling and thrombus stability in TSSC6-deficient mice. Blood 108, 1911–1918 (2006).
Israels, S. J. & McMillan-Ward, E. M. CD63 modulates spreading and tyrosine phosphorylation of platelets on immobilized fibrinogen. Thromb. Haemost. 93, 311–318 (2005).
Tricoci, P. & Peterson, E. D. The evolving role of glycoprotein IIb/IIIa inhibitor therapy in contemporary care of acute coronary syndrome patients. J. Interv. Cardiol. 19, 449–455 (2006).
Moseley, G. W. Tetraspanin–Fc receptor interactions. Platelets 16, 3–12 (2005).
Cowin, A. J. et al. Wound healing is defective in mice lacking tetraspanin CD151. J. Invest. Dermatol. 126, 680–689 (2006).
Dijkstra, S. et al. Intraspinal administration of an antibody against CD81 enhances functional recovery and tissue sparing after experimental spinal cord injury. Exp. Neurol. 202, 57–66 (2006). One of the few examples of an anti-tetraspanin mAb used successfully in vivo in a disease model.
Garcia, E. et al. HIV-1 trafficking to the dendritic cell–T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6, 488–501 (2005).
Wiley, R. D. & Gummuluru, S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl Acad. Sci. USA 103, 738–743 (2006).
Gordon-Alonso, M. et al. Tetraspanins CD9 and CD81 modulate HIV-1-induced membrane fusion. J. Immunol. 177, 5129–5137 (2006).
von Lindern J. J. et al. Potential role for CD63 in CCR5-mediated human immunodeficiency virus type 1 infection of macrophages. J. Virol. 77, 3624–3633 (2003).
Ho, S. H. et al. Recombinant extracellular domains of tetraspanin proteins are potent inhibitors of the infection of macrophages by human immunodeficiency virus type 1. J. Virol. 80, 6487–6496 (2006).
Deneka, M., Pelchen-Matthews, A., Byland, R., Ruiz-Mateos, E. & Marsh, M. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J. Cell Biol. 177, 329–341 (2007).
Pelchen-Matthews, A., Kramer, B. & Marsh, M. Infectious HIV-1 assembles in late endosomes in primary macrophages. J. Cell Biol. 162, 443–455 (2003).
Ruiz-Mateos, E., Pelchen-Matthews, A., Deneka, M. & Marsh, M. CD63 is not required for the production of infectious human immunodeficiency virus type 1 in human macrophages. J. Virol. 82, 4751–4761 (2008).
Sato, K. et al. Modulation of human immunodeficiency virus type 1 infectivity through incorporation of tetraspanin proteins. J. Virol. 82, 1021–1033 (2008).
Cocquerel, L., Voisset, C. & Dubuisson, J. Hepatitis C virus entry: potential receptors and their biological functions. J. Gen. Virol. 87, 1075–1084 (2006).
Flint, M. et al. Diverse CD81 proteins support hepatitis C virus infection. J. Virol. 80, 11331–11342 (2006).
Bertaux, C. & Dragic, T. Different domains of CD81 mediate distinct stages of hepatitis C virus pseudoparticle entry. J. Virol. 80, 4940–4948 (2006).
Molina, S. et al. Serum-derived hepatitis C virus infection of primary human hepatocytes is tetraspanin CD81 dependent. J. Virol. 82, 569–574 (2008).
Harris, H. J. et al. CD81 and Claudin 1 co-receptor association: a role in hepatitis C virus entry. J. Virol. 82, 5007-5020 (2008).
Evans, M. J. et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446, 801–805 (2007).
Yang, W. et al. Correlation of the tight junction-like distribution of Claudin-1 to the cellular tropism of hepatitis C virus. J. Biol. Chem. 283, 8643–8653 (2008).
Fukudome, K. et al. Identification of membrane antigen C33 recognized by monoclonal antibodies inhibitory to human T-cell leukemia virus type 1 (HTLV- 1)-induced syncytium formation: altered glycosylation of C33 antigen in HTLV-1-positive T cells. J. Virol. 66, 1394–1401 (1992).
Pique, C. et al. Interaction of CD82 tetraspanin proteins with HTLV-1 envelope glycoproteins inhibits cell-to-cell fusion and virus transmission. Virology 276, 455–465 (2000).
Mazurov, D., Heidecker, G. & Derse, D. The inner loop of tetraspanins CD82 and CD81 mediates interactions with human T cell lymphotrophic virus type 1 Gag protein. J. Biol. Chem. 282, 3896–3903 (2007).
Loffler, S. et al. CD9, a tetraspan transmembrane protein, renders cells susceptible to canine distemper virus. J. Virol. 71, 42–49 (1997).
Singethan, K. et al. CD9-dependent regulation of Canine distemper virus-induced cell-cell fusion segregates with the extracellular domain of the haemagglutinin. J. Gen. Virol. 87, 1635–1642 (2006).
Tachibana, I. & Hemler, M. E. Role of transmembrane-4 superfamily (TM4SF) proteins CD9 and CD81 in muscle cell fusion and myotube maintenance. J. Cell Biol. 146, 893–904 (1999).
Tanio, Y., Yamazaki, H., Kunisada, T., Miyake, K. & Hayashi, S. I. CD9 molecule expressed on stromal cells is involved in osteoclastogenesis. Exp. Hematol. 27, 853–859 (1999).
Takeda, Y. et al. Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes. J. Cell Biol. 161, 945–956 (2003).
de Parseval, A., Lerner, D. L., Borrow, P., Willett, B. & Elder, J. H. Blocking of feline immunodeficiency virus infection by a monoclonal antibody to CD9 is via inhibition of virus release rather than interference with receptor binding. J. Virol. 71, 5742–5749 (1997).
Silvie, O. et al. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nature Med. 9, 93–96 (2003).
Iwamoto, R. et al. Heparin-binding EGF-like growth factor, which acts as a diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which upregulates functional receptors and diphtheria toxin sensitivity. EMBO J. 13, 2322–2330 (1994).
Reichert, J. M. & Valge-Archer, V. E. Development trends for monoclonal antibody cancer therapeutics. Nature Rev. Drug Discov. 6, 349–356 (2007).
Liu, W. M. et al. Tetraspanin CD9 regulates invasion during mouse embryo implantation. J. Mol. Endocrinol. 36, 121–130 (2006).
Zhao, X. et al. Targeting CD37-positive lymphoid malignancies with a novel engineered small modular immunopharmaceutical. Blood 110, 2569–2577 (2007). One of the few examples of successful tetraspanin targeting in vivo.
Levy, S., Todd, S. C. & Maecker, H. T. CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu. Rev. Immunol. 16, 89–109 (1998).
Oren, R., Takahashi, S., Doss, C., Levy, R. & Levy, S. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol. Cell Biol. 10, 4007–4015 (1990).
Boismenu, R., Rhein, M., Fischer, W. H. & Havran, W. L. A role for CD81 in early T cell development. Science 271, 198–200 (1996).
Tsitsikov, E. N., Gutierrez-Ramos, J. C. & Geha, R. S. Impaired CD19 expression and signaling, enhanced antibody response to type II T independent antigen and reduction of B-1 cells in CD81-deficient mice. Proc. Natl Acad. Sci. USA 94, 10844–10849 (1997).
Geary, S. M., Cambareri, A. C., Sincock, P. M., Fitter, S. & Ashman, L. K. Differential tissue expression of epitopes of the tetraspanin CD151 recognised by monoclonal antibodies. Tissue Antigens 58, 141–153 (2001).
Yauch, R. L., Kazarov, A. R., Desai, B., Lee, R. T. & Hemler, M. E. Direct extracellular contact between integrin α3β1 and TM4SF protein CD151. J. Biol. Chem. 275, 9230–9238 (2000).
Serru, V. et al. Selective tetraspan-integrin complexes (CD81/α4β1, CD151/α3β1, CD151/α6β1) under conditions disrupting tetraspan interactions. Biochem. J. 340, 103–111 (1999).
Kolesnikova, T. V. et al. EWI-2 modulates lymphocyte integrin a4b1 functions. Blood 103, 3013–3019 (2004).
Gutierrez-Lopez, M. D. et al. A functionally relevant conformational epitope on the CD9 tetraspanin depends on the association with activated β1 integrin. J. Biol. Chem. 278, 208–218 (2003).
Yang, X. H. et al. Contrasting Effects of EWI proteins, integrins, and protein palmitoylation on cell surface CD9 organization. J. Biol. Chem. 281, 12976–12985 (2006).
Murayama, Y. et al. CD9-mediated activation of the p46 Shc isoform leads to apoptosis in cancer cells. J. Cell Sci. 117, 3379–3388 (2004).
Press, O. W. et al. Treatment of refractory non-Hodgkin's lymphoma with radiolabeled MB-1 (anti-CD37) antibody. J. Clin. Oncol. 7, 1027–1038 (1989).
Zhu, G. Z. et al. Residues SFQ (173–175) in the large extracellular loop of CD9 are required for gamete fusion. Development 129, 1995–2002 (2002).
Higginbottom, A. et al. Structural requirements for the inhibitory action of the CD9 large extracellular domain in sperm/oocyte binding and fusion. Biochem. Biophys. Res. Commun. 311, 208–214 (2003).
Barreiro, O. et al. Endothelial tetraspanin microdomains regulate leukocyte firm adhesion during extravasation. Blood 105, 2852–2861 (2005).
Winterwood, N. E., Varzavand, A., Meland, M. N., Ashman, L. K. & Stipp, C. S. A critical role for tetraspanin CD151 in α3β1 and α6β4 integrin-dependent tumor cell functions on laminin-5. Mol. Biol. Cell 17, 2707–2721 (2006).
Furuya, M. et al. Down-regulation of CD9 in human ovarian carcinoma cell might contribute to peritoneal dissemination: morphologic alteration and reduced expression of β1 integrin subsets. Cancer Res. 65, 2617–2625 (2005).
Iwai, K., Ishii, M., Ohshima, S., Miyatake, K. & Saeki, Y. Expression and function of transmembrane-4 superfamily (tetraspanin) proteins in osteoclasts: reciprocal roles of Tspan-5 and NET-6 during osteoclastogenesis. Allergol. Int. 56, 457–463 (2007).
Shanmukhappa, K., Kim, J. K. & Kapil, S. Role of CD151, A tetraspanin, in porcine reproductive and respiratory syndrome virus infection. Virol. J. 4, 62 (2007).
Bahi, A., Boyer, F., Kolira, M. & Dreyer, J. L. In vivo gene silencing of CD81 by lentiviral expression of small interference RNAs suppresses cocaine-induced behaviour. J. Neurochem. 92, 1243–1255 (2005).
de Fougerolles, A., Vornlocher, H. P., Maraganore, J. & Lieberman, J. Interfering with disease: a progress report on siRNA-based therapeutics. Nature Rev. Drug Discov. 6, 443–453 (2007).
Peer, D., Park, E. J., Morishita, Y., Carman, C. V. & Shimaoka, M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science 319, 627–630 (2008).
Cannon, K. S. & Cresswell, P. Quality control of transmembrane domain assembly in the tetraspanin CD82. EMBO J. 20, 2443–2453 (2001).
Goldberg, A. F. et al. An intramembrane glutamic acid governs peripherin/rds function for photoreceptor disk morphogenesis. Invest. Ophthalmol. Vis. Sci. 48, 2975–2986 (2007).
Tarasova, N. I., Rice, W. G. & Michejda, C. J. Inhibition of G-protein-coupled receptor function by disruption of transmembrane domain interactions. J. Biol. Chem. 274, 34911–34915 (1999).
Latysheva, N. et al. Syntenin-1 is a new component of tetraspanin-enriched microdomains: mechanisms and consequences of the interaction of syntenin-1 with CD63. Mol. Cell Biol. 26, 7707–7718 (2006). First demonstration of a specific tetraspanin interaction with a PDZ domain-containing protein.
Dev., K. K. Making protein interactions druggable: targeting PDZ domains. Nature Rev. Drug Discov. 3, 1047–1056 (2004).
Holzer, M. et al. Identification of terfenadine as an inhibitor of human CD81-receptor HCV-E2 interaction: synthesis and structure optimization. Molecules 13, 1081–1110 (2008).
VanCompernolle, S. E. et al. Small molecule inhibition of hepatitis C virus E2 binding to CD81. Virology 314, 371–380 (2003).
Mitchell, D. A., Vasudevan, A., Linder, M. E. & Deschenes, R. J. Protein palmitoylation by a family of DHHC protein S-acyltransferases. J. Lipid Res. 47, 1118–1127 (2006).
Sharma, C., Yang, X. H. & Hemler, M. E. DHHC2 affects palmitoylation and stability of tetraspanins CD9 and CD151. Mol. Biol. Cell 19, 3415–3425 (2008).
Tsai, Y. C. et al. The ubiquitin ligase gp78 promotes sarcoma metastasis by targeting KAI1 for degradation. Nature Med. 13, 1504–1509 (2007).
Kurzeder, C. et al. CD9 promotes adeno-associated virus type 2 infection of mammary carcinoma cells with low cell surface expression of heparan sulphate proteoglycans. Int. J. Mol. Med. 19, 325–333 (2007).
Zhang, X. A., Lane, W. S., Charrin, S., Rubinstein, E. & Liu, L. EWI2/PGRL associates with the metastasis suppressor KAI1/CD82 and inhibits the migration of prostate cancer cells. Cancer Res. 63, 2665–2674 (2003).
Rocha-Perugini, V. et al. The CD81 partner EWI-2wint inhibits hepatitis C virus entry. PLoS ONE 3, e1866 (2008).
Higashiyama, S. et al. The membrane protein CD9/DRAP27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J. Cell Biol. 128, 929–938 (1995).
Inui, S. et al. Possible role of coexpression of CD9 with membrane-anchored heparin-binding EGF-like growth factor and amphiregulin in cultured human keratinocyte growth. J. Cell Physiol. 171, 291–298 (1997).
Shi, W., Fan, H., Shum, L. & Derynck, R. The tetraspanin CD9 associates with transmembrane TGF-α and regulates TGF-α-induced EGF receptor activation and cell proliferation. J. Cell Biol. 148, 591–602 (2000).
Yan, Y., Shirakabe, K. & Werb, Z. The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G. protein-coupled receptors. J. Cell Biol. 158, 221–226 (2002).
Fearon, D. T. & Carter, R. H. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural and acquired immunity. Annu. Rev. Immunol. 13, 127–149 (1995).
Sterk, L. M. et al. Association of the tetraspanin CD151 with the laminin-binding integrins α3β1, α6β1, α6β4 and α7β1 in cells in culture and in vivo. J. Cell Sci. 115, 1161–1173 (2002). Excellent use of diagnostic anti-CD151 antibodies to distinguish integrin-associated and non-associated CD151.
Sincock, P. M. et al. PETA-3/CD151, a member of the transmembrane 4 superfamily, is localised to the plasma membrane and endocytic system of endothelial cells, associates with multiple integrins and modulates cell function. J. Cell Sci. 112, 833–844 (1999).
Yanez-Mo, M. et al. Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-3 with α3β1 integrin localized at endothelial lateral junctions. J. Cell Biol. 141, 791–804 (1998).
Zhang, X. A. et al. Function of the tetraspanin CD151–α6β1 integrin complex during cellular morphogenesis. Mol. Biol. Cell 13, 1–11 (2002).
Lammerding, J., Kazarov, A. R., Huang, H., Lee, R. T. & Hemler, M. E. Tetraspanin CD151 regulates α6β1 integrin adhesion strengthening. Proc. Natl Acad. Sci. USA 100, 7616–7621 (2003). Ligand-coated magnetic beads used to show that CD151 affects ligand detachment rather than attachment.
Stipp, C. S., Kolesnikova, T. V. & Hemler, M. E. EWI-2 is a major CD9 and CD81 partner, and member of a novel Ig protein subfamily. J. Biol. Chem. 276, 40545–40554 (2001).
Stipp, C. S., Orlicky, D. & Hemler, M. E. FPRP: A major, highly stoichiometric, highly specific CD81 and CD9-associated protein. J. Biol. Chem. 276, 4853–4862 (2001).
Charrin, S. et al. The major CD9 and CD81 molecular partner. Identification and characterization of the complexes. J. Biol. Chem. 276, 14329–14337 (2001).
Clark, K. L., Zeng, Z., Langford, A. L., Bowen, S. M. & Todd, S. C. Pgrl is a major CD81-associated protein on lymphocytes and distinguishes a new family of cell surface proteins. J. Immunol. 167, 5115–5121 (2001).
Horvath, G. et al. CD19 is linked to the integrin-associated tetraspans CD9, CD81, and CD82. J. Biol. Chem. 273, 30537–30543 (1998).
Yauch, R. L., Berditchevski, F., Harler, M. B., Reichner, J. & Hemler, M. E. Highly stoichiometric, stable and specific association of integrin α3β1 with CD151 provides a major link to phosphatidylinositol 4-kinase and may regulate cell migration. Mol. Biol. Cell 9, 2751–2765 (1998).
Miyado, K. et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science 287, 321–324 (2000).
Le Naour, F., Rubinstein, E., Jasmin, C., Prenant, M. & Boucheix, C. Severely reduced female fertility in CD9-deficient mice. Science 287, 319–321 (2000).
Kaji, K. et al. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nature Genet. 24, 279–282 (2000).
Kaminski, M. S. et al. Imaging, dosimetry, and radioimmunotherapy with iodine 131-labeled anti-CD37 antibody in B-cell lymphoma. J. Clin. Oncol. 10, 1696–1711 (1992).
Tanigawa, M. et al. Possible involvement of CD81 in acrosome reaction of sperm in mice. Mol. Reprod. Dev. 75, 150–155 (2008).
Schmid, E. et al. Antibodies to CD9, a tetraspan transmembrane protein, inhibit canine distemper virus-induced cell–cell fusion but not virus–cell fusion. J. Virol. 74, 7554–7561 (2000).
Iwamoto, R., Senoh, H., Okada, Y., Uchida, T. & Mekada, E. An antibody that inhibits the binding of diphtheria toxin to cells revealed the association of a 27-kD membrane protein with the diphtheria toxin receptor. J. Biol. Chem. 266, 20463–20469 (1991).
Beatty, W. L. Trafficking from CD63-positive late endocytic multivesicular bodies is essential for intracellular development of Chlamydia trachomatis. J. Cell Sci. 119, 350–359 (2006).
Acknowledgements
The author gratefully acknowledges support from the National Institutes of Health grants GM38903 and CA42368.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Integrins
-
A family of cell-surface transmembrane proteins (24 mammalian members), with αβ-heterodimeric structures, which function as cell adhesion molecules.
- EWI proteins
-
A family of four cell-surface immunoglobulin superfamily proteins, sharing a conserved glutamine-tryptophan-isoleucine (EWI) motif.
- Protein palmitoylation
-
Post-translational acylation of a protein, typically on an intracellular cysteine residue.
- Exosomes
-
Vesicles of 50–100 nm, enriched 10-fold to 100-fold for tetraspanins, and shed from the multivesicular bodies of cells.
- Monoclonal antibody
-
A specific antibody produced in large quantity by a single hybrid cell clone formed in the laboratory by the fusion of a B cell with a tumour cell.
- Angiogenesis
-
The process by which new blood vessels grow from pre-existing blood vessels.
- RNA interference
-
(RNAi). A form of post-transcriptional gene silencing in which expression or transfection of dsRNA induces degradation, by nucleases, of the homologous endogenous transcripts, resulting in reduction or loss of gene activity.
- DHHC2
-
A member of a family of enzymes (24 members in mammals) containing a conserved aspartate-histidine-histidine-cysteine (DHHC) motif, responsible for the S-palmitoylation of proteins.
Rights and permissions
About this article
Cite this article
Hemler, M. Targeting of tetraspanin proteins — potential benefits and strategies. Nat Rev Drug Discov 7, 747–758 (2008). https://doi.org/10.1038/nrd2659
Issue Date:
DOI: https://doi.org/10.1038/nrd2659