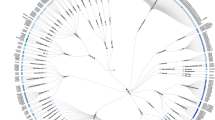
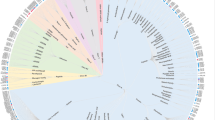
Key Points
-
The elucidation of the primary structure of the first identified G-protein-coupled receptor (GPCR) rhodopsin 25 years ago was a key step in the development of the concept of a GPCR superfamily. Now, data from our recent mining of the human genome suggests that humans have at least 799 full-length GPCRs, making them the largest superfamily of membrane-bound receptors.
-
Members of the GPCR superfamily are diverse in their primary structure, and classification of these receptors has been made on the basis of phylogenetic criteria. This showed that most of the human GPCRs can be grouped into five main families: Glutamate, Rhodopsin, Adhesion, Frizzled/Taste2 and Secretin.
-
The Rhodopsin family is the largest family of GPCRs, containing ∼670 full-length human receptor proteins. They can be divided into four groups and bind to a wide range of ligands. Their diversity is attributed to differences within the transmembrane regions. So far, the only crystal structures available for the transmembrane regions of GPCRs are the bovine rhodopsin receptor and the human β2-adrenoceptor (ADRB2). These two structures have aided the understanding of how GPCRs are activated and in homology modelling.
-
The Adhesion family is the second largest and can be subdivided into eight subgroups. They are rich in functional domains and most have long and diverse N termini. The N termini may contain domains that are also found in other proteins (for example, lectin, cadherin), and the number and structure of these domains has been shown to have an important role in the specificity of the receptor–ligand binding interactions.
-
The Secretin family is small and share between 21–67% sequence identity. They all have an extracellular hormone-binding domain and bind peptide hormones.
-
The Glutamate family consists of 22 human proteins, and most members bind to their respective endogenous ligand within the N-terminal region.
-
The frizzled receptors bind the family of Wnt glycoproteins and are implicated in cancer development. The Taste2 receptors can be divided into five subgroups and have great sequence diversity. This diversity may explain how a limited number of receptors can distinguish between the thousands of bitter compounds that humans can detect.
-
GPCRs are one of most common targets of therapeutic drugs so far, with drugs available that target at least 46 receptors in three of the families: Rhodopsin, Secretin and Glutamate. It is proposed that 323 GPCRs could also represent drug targets, and ∼150 are still orphans.
Abstract
G protein-coupled receptors (GPCRs) are the largest family of membrane-bound receptors and also the targets of many drugs. Understanding of the functional significance of the wide structural diversity of GPCRs has been aided considerably in recent years by the sequencing of the human genome and by structural studies, and has important implications for the future therapeutic potential of targeting this receptor family. This article aims to provide a comprehensive overview of the five main human GPCR families — Rhodopsin, Secretin, Adhesion, Glutamate and Frizzled/Taste2 — with a focus on gene repertoire, general ligand preference, common and unique structural features, and the potential for future drug discovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nathans, J. & Hogness, D. S. Isolation, sequence analysis, and intron–exon arrangement of the gene encoding bovine rhodopsin. Cell 34, 807–814 (1983).
Hargrave, P. A. et al. The structure of bovine rhodopsin. Biophys. Struct. Mech. 9, 235–244 (1983).
Hargrave, P. A. et al. Rhodopsin's protein and carbohydrate structure: selected aspects. Vision Res. 24, 1487–1499 (1984).
Lefkowitz, R. J. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol. Sci. 25, 413–422 (2004).
Dixon, R. A. et al. Cloning of the gene and cDNA for mammalian β-adrenergic receptor and homology with rhodopsin. Nature 321, 75–79 (1986).
Kobilka, B. K. et al. Functional activity and regulation of human β2-adrenergic receptors expressed in Xenopus oocytes. J. Biol. Chem. 262, 15796–15802 (1987).
Felder, C. C. et al. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc. Natl Acad. Sci. USA 90, 7656–7660 (1993).
Masu, Y. et al. cDNA cloning of bovine substance-K receptor through oocyte expression system. Nature 329, 836–838 (1987).
Parmentier, M. et al. Molecular cloning of the thyrotropin receptor. Science 246, 1620–1622 (1989).
Attwood, T. K. & Findlay, J. B. Design of a discriminating fingerprint for G-protein-coupled receptors. Protein Eng. 6, 167–176 (1993).
Attwood, T. K. & Findlay, J. B. Fingerprinting G-protein-coupled receptors. Protein Eng. 7, 195–203 (1994).
Kolakowski, L. F. Jr. GCRDb: a G-protein-coupled receptor database. Receptors Channels 2, 1–7 (1994).
Foord, S. M. et al. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol. Rev. 57, 279–288 (2005). The NC-IUPHAR committee is central for improving the nomenclature within the GPCR field. This paper provides the reader with an updated list of the human GPCRs, their ligands and appropriate abbreviations.
Bockaert, J. & Pin, J. P. Molecular tinkering of G protein-coupled receptors: an evolutionary success. Embo J. 18, 1723–1729 (1999).
Lander, E. S. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001).
Venter, J. C. et al. The sequence of the human genome. Science 291, 1304–1351 (2001).
Vassilatis, D. K. et al. The G protein-coupled receptor repertoires of human and mouse. Proc. Natl Acad. Sci. USA 100, 4903–4908 (2003).
Fredriksson, R., Lagerstrom, M. C., Lundin, L. G. & Schioth, H. B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63, 1256–1272 (2003). This articles introduces the concept of the five main families of GPCRs: Glutamate, Rhodopsin, Adhesion, Frizzled/Taste2 and Secretin . The paper subdivides the Rhodopsin family into four groups and divides the former class B into two different families based on sequence comparison (phylogeny) and also introduces the bitter taste2 receptors into the nomenclature system.
Adler, E. et al. A novel family of mammalian taste receptors. Cell 100, 693–702 (2000).
Matsunami, H., Montmayeur, J. P. & Buck, L. B. A family of candidate taste receptors in human and mouse. Nature 404, 601–604 (2000).
Fredriksson, R. & Schioth, H. B. The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol. Pharmacol. 67, 1414–1425 (2005).
Gloriam, D. E., Fredriksson, R. & Schioth, H. B. The G protein-coupled receptor subset of the rat genome. BMC Genomics 8, 338–405 (2007). This paper provides the currently most up-to-date list of human, mouse and rat repertoires of GPCRs.
Lagerstrom, M. C. et al. The G protein-coupled receptor subset of the chicken genome. PLoS Comput. Biol. 2, e54 (2006).
Palczewski, K. et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 (2000). This report of the first crystallized GPCR, the bovine rhodopsin receptor, has been vital for understanding the three-dimensional structure of a GPCR. The information has been used extensively for homology modelling and the article has so far been cited over 2,000 times.
Schwartz, T. W. Locating ligand-binding sites in 7TM receptors by protein engineering. Curr. Opin. Biotechnol. 5, 434–444 (1994).
Frimurer, T. M. et al. A physicogenetic method to assign ligand-binding relationships between 7TM receptors. Bioorg. Med. Chem. Lett. 15, 3707–3712 (2005).
Cherezov, V. et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein coupled receptor. Science 318, 1258–1265 (2007). Seven years after the crystallization of rhodopsin, the next GPCR structure was reported. The high-resolution structure of the β 2 -adrenergic receptor provides the field with the first coordinates of a GPCR interacting with diffusible ligands.
Rasmussen, S. G. et al. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature 450, 383–387 (2007).
Vu, T. K., Hung., D. T., Wheaton, V. I. & Coughlin, S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 64, 1057–1068 (1991).
Xu, W. F. et al. Cloning and characterization of human protease-activated receptor 4. Proc. Natl Acad. Sci. USA 95, 6642–6646 (1998).
Nystedt, S., Emilsson, K., Larsson, A. K., Strombeck, B. & Sundelin, J. Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur. J. Biochem. 232, 84–89 (1995).
Ishihara, H. et al. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature 386, 502–506 (1997).
Oikonomopoulou, K. et al. Proteinase-mediated cell signalling: targeting proteinase-activated receptors (PARs) by kallikreins and more. Biol. Chem. 387, 677–685 (2006).
Hsu, S. Y. et al. The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol. Endocrinol. 14, 1257–1271 (2000).
Hsu, S. Y. et al. Activation of orphan receptors by the hormone relaxin. Science 295, 671–674 (2002).
Fan, Q. R. & Hendrickson, W. A. Structure of human follicle-stimulating hormone in complex with its receptor. Nature 433, 269–277 (2005).
Tyndall, J. D. & Sandilya, R. GPCR agonists and antagonists in the clinic. Med. Chem. 1, 405–421 (2005). This review summarizes clinically available drugs that target GPCRs.
Surgand, J. S., Rodrigo, J., Kellenberger, E. & Rognan, D. A chemogenomic analysis of the transmembrane binding cavity of human G-protein-coupled receptors. Proteins 62, 509–538 (2006).
Jacoby, E., Bouhelal, R., Gerspacher, M. & Seuwen, K. The 7 TM G-protein-coupled receptor target family. ChemMedChem 1, 761–782 (2006).
Strader, C. D., Sigal, I. S. & Dixon, R. A. Structural basis of β-adrenergic receptor function. Faseb J. 3, 1825–1832 (1989).
Liapakis, G. et al. The forgotten serine. A critical role for Ser-2035.42 in ligand binding to and activation of the β2-adrenergic receptor. J. Biol. Chem. 275, 37779–37788 (2000).
Swaminath, G. et al. Sequential binding of agonists to the β2 adrenoceptor. Kinetic evidence for intermediate conformational states. J. Biol. Chem. 279, 686–691 (2004).
Van Gaal, L. F., Rissanen, A. M., Scheen, A. J., Ziegler, O. & Rossner, S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365, 1389–1397 (2005).
Onuffer, J. J. & Horuk, R. Chemokines, chemokine receptors and small-molecule antagonists: recent developments. Trends Pharmacol. Sci. 23, 459–467 (2002).
Civelli, O., Saito, Y., Wang, Z., Nothacker, H. P. & Reinscheid, R. K. Orphan GPCRs and their ligands. Pharmacol. Ther. 110, 525–532 (2006).
Overton, H. A. et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell. Metab. 3, 167–175 (2006).
Fredriksson, R., Hoglund, P. J., Gloriam, D. E., Lagerstrom, M. C. & Schioth, H. B. Seven evolutionarily conserved human rhodopsin G protein-coupled receptors lacking close relatives. FEBS Lett. 554, 381–388 (2003).
Hirasawa, A. et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nature Med. 11, 90–94 (2005).
Thomas, P., Pang, Y., Filardo, E. J. & Dong, J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146, 624–632 (2005).
Wang, J. et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 281, 22021–22028 (2006).
Tabata, K., Baba, K., Shiraishi, A., Ito, M. & Fujita, N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. 363, 861–866 (2007).
Bjarnadottir, T. K. et al. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 88, 263–273 (2006).
Levoye, A. et al. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. Embo J. 25, 3012–3023 (2006).
Galvez, T. et al. Allosteric interactions between GB1 and GB2 subunits are required for optimal GABAB receptor function. Embo J. 20, 2152–2159 (2001).
Jones, K. A. et al. GABAB receptors function as a heteromeric assembly of the subunits GABABR1 and GABABR2. Nature 396, 674–679 (1998).
Robbins, M. J. et al. GABAB2 is essential for G-protein coupling of the GABAB receptor heterodimer. J. Neurosci. 21, 8043–8052 (2001).
Hoon, M. A. et al. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell 96, 541–551 (1999).
Li, X. et al. Human receptors for sweet and umami taste. Proc. Natl Acad. Sci. USA 99, 4692–4696 (2002).
Nelson, G. et al. An amino-acid taste receptor. Nature 416, 199–202 (2002).
Nelson, G. et al. Mammalian sweet taste receptors. Cell 106, 381–390 (2001).
Sainz, E., Korley, J. N., Battey, J. F. & Sullivan, S. L. Identification of a novel member of the T1R family of putative taste receptors. J. Neurochem. 77, 896–903 (2001).
Libert, F. et al. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science 244, 569–572 (1989).
Buck, L. & Axel, R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175–187 (1991).
Parmentier, M. et al. Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature 355, 453–455 (1992).
Mombaerts, P. The human repertoire of odorant receptor genes and pseudogenes. Annu. Rev. Genomics Hum. Genet. 2, 493–510 (2001).
Malnic, B., Godfrey, P. A. & Buck, L. B. The human olfactory receptor gene family. Proc. Natl Acad. Sci. USA 101, 2584–2589 (2004).
Niimura, Y. & Nei, M. Evolution of olfactory receptor genes in the human genome. Proc. Natl Acad. Sci. USA 100, 12235–12240 (2003).
Zozulya, S., Echeverri, F. & Nguyen, T. The human olfactory receptor repertoire. Genome Biol. 2, Research0018.1–0018.12 (2001).
Glusman, G. et al. The olfactory receptor gene superfamily: data mining, classification, and nomenclature. Mamm. Genome 11, 1016–1023 (2000).
Rovati, G. E., Capra, V. & Neubig, R. R. The highly conserved DRY motif of class A G protein-coupled receptors: beyond the ground state. Mol. Pharmacol. 71, 959–964 (2007).
Man, O., Gilad, Y. & Lancet, D. Prediction of the odorant binding site of olfactory receptor proteins by human–mouse comparisons. Protein Sci. 13, 240–254 (2004).
Floriano, W. B., Vaidehi, N., Goddard, W. A. 3rd, Singer, M. S. & Shepherd, G. M. Molecular mechanisms underlying differential odor responses of a mouse olfactory receptor. Proc. Natl Acad. Sci. USA 97, 10712–10716 (2000).
Gaillard, I., Rouquier, S., Chavanieu, A., Mollard, P. & Giorgi, D. Amino-acid changes acquired during evolution by olfactory receptor 912–93 modify the specificity of odorant recognition. Hum. Mol. Genet. 13, 771–780 (2004).
Singer, M. S. Analysis of the molecular basis for octanal interactions in the expressed rat 17 olfactory receptor. Chem. Senses 25, 155–165 (2000).
Malnic, B., Hirono, J., Sato, T. & Buck, L. B. Combinatorial receptor codes for odors. Cell 96, 713–723 (1999). A seminal paper describing the concept of combinatorial receptor codes for different odorants, which enables humans to distinguish a vast number of odorants from each other.
Wetzel, C. H. et al. Specificity and sensitivity of a human olfactory receptor functionally expressed in human embryonic kidney 293 cells and Xenopus laevis oocytes. J. Neurosci. 19, 7426–7433 (1999).
Spehr, M. et al. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 299, 2054–2058 (2003).
Shirokova, E. et al. Identification of specific ligands for orphan olfactory receptors. G protein-dependent agonism and antagonism of odorants. J. Biol. Chem. 280, 11807–11815 (2005).
Krautwurst, D., Yau, K. W. & Reed, R. R. Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell 95, 917–926 (1998).
Matarazzo, V. et al. Functional characterization of two human olfactory receptors expressed in the baculovirus sf9 insect cell system. Chem. Senses 30, 195–207 (2005).
Young, J. M. & Trask, B. J. The sense of smell: genomics of vertebrate odorant receptors. Hum. Mol. Genet. 11, 1153–1160 (2002).
Harmar, A. J. Family-B G-protein-coupled receptors. Genome Biol. 2, Reviews3013.1–3113.10 (2001).
Ishihara, T. et al. Molecular cloning and expression of a cDNA encoding the secretin receptor. Embo J. 10, 1635–1641 (1991).
Hofmann, B. A. et al. Functional and protein chemical characterization of the N-terminal domain of the rat corticotropin-releasing factor receptor 1. Protein Sci. 10, 2050–2062 (2001).
Grauschopf, U. et al. The N-terminal fragment of human parathyroid hormone receptor 1 constitutes a hormone binding domain and reveals a distinct disulfide pattern. Biochemistry 39, 8878–8887 (2000).
Bazarsuren, A. et al. In vitro folding, functional characterization, and disulfide pattern of the extracellular domain of human GLP-1 receptor. Biophys. Chem. 96, 305–318 (2002).
Grace, C. R. et al. NMR structure and peptide hormone binding site of the first extracellular domain of a type B1 G protein-coupled receptor. Proc. Natl Acad. Sci. USA 101, 12836–12841 (2004).
Godfrey, P. et al. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nature Genet. 4, 227–232 (1993).
DeAlmeida, V. I. & Mayo, K. E. Identification of binding domains of the growth hormone-releasing hormone receptor by analysis of mutant and chimeric receptor proteins. Mol. Endocrinol. 12, 750–765 (1998).
Assil, I. Q., Qi, L. J., Arai, M., Shomali, M. & Abou-Samra, A. B. Juxtamembrane region of the amino terminus of the corticotropin releasing factor receptor type 1 is important for ligand interaction. Biochemistry 40, 1187–1195 (2001).
Vilardaga, J. P., Lin, I. & Nissenson, R. A. Analysis of parathyroid hormone (PTH)/secretin receptor chimeras differentiates the role of functional domains in the pth/ pth-related peptide (PTHrP) receptor on hormone binding and receptor activation. Mol. Endocrinol. 15, 1186–1199 (2001).
Unson, C. G. et al. Roles of specific extracellular domains of the glucagon receptor in ligand binding and signaling. Biochemistry 41, 11795–11803 (2002).
Pham, V., Wade, J. D., Purdue, B. W. & Sexton, P. M. Spatial proximity between a photolabile residue in position 19 of salmon calcitonin and the amino terminus of the human calcitonin receptor. J. Biol. Chem. 279, 6720–6729 (2004).
Dong, M. et al. Spatial approximation between two residues in the mid-region of secretin and the amino terminus of its receptor. Incorporation of seven sets of such constraints into a three-dimensional model of the agonist-bound secretin receptor. J. Biol. Chem. 278, 48300–48312 (2003).
Dong, M., Pinon, D. I., Cox, R. F. & Miller, L. J. Importance of the amino terminus in secretin family G protein-coupled receptors. Intrinsic photoaffinity labeling establishes initial docking constraints for the calcitonin receptor. J. Biol. Chem. 279, 1167–1175 (2004).
Dong, M., Li, Z., Pinon, D. I., Lybrand, T. P. & Miller, L. J. Spatial approximation between the amino terminus of a peptide agonist and the top of the sixth transmembrane segment of the secretin receptor. J. Biol. Chem. 279, 2894–2903 (2004).
Nauck, M. A. & Meier, J. J. Glucagon-like peptide 1 and its derivatives in the treatment of diabetes. Regul. Pept. 128, 135–148 (2005).
Stacey, M., Lin, H. H., Gordon, S. & McKnight, A. J. LNB-TM7, a group of seven-transmembrane proteins related to family-B G-protein-coupled receptors. Trends Biochem. Sci. 25, 284–289 (2000).
Bjarnadottir, T. K. et al. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics 84, 23–33 (2004). This paper highlighted the diversity of functional domains within the Adhesion family of GPCRs.
Baud, V. et al. EMR1, an unusual member in the family of hormone receptors with seven transmembrane segments. Genomics 26, 334–344 (1995).
Krasnoperov, V. G. et al. α-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron 18, 925–937 (1997).
Krasnoperov, V. et al. Post-translational proteolytic processing of the calcium-independent receptor of α-latrotoxin (CIRL), a natural chimera of the cell adhesion protein and the G protein-coupled receptor. Role of the G protein-coupled receptor proteolysis site (GPS) motif. J. Biol. Chem. 277, 46518–46526 (2002).
Lin, H. H. et al. Autocatalytic cleavage of the EMR2 receptor occurs at a conserved G protein-coupled receptor proteolytic site motif. J. Biol. Chem. 279, 31823–31832 (2004).
Nechiporuk, T., Urness, L. D. & Keating, M. T. ETL, a novel seven-transmembrane receptor that is developmentally regulated in the heart. ETL is a member of the secretin family and belongs to the epidermal growth factor-seven-transmembrane subfamily. J. Biol. Chem. 276, 4150–4157 (2001).
Lin, H. H. et al. Molecular analysis of the epidermal growth factor-like short consensus repeat domain-mediated protein–protein interactions: dissection of the CD97–CD55 complex. J. Biol. Chem. 276, 24160–24169 (2001).
Lelianova, V. G. et al. α-Latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J. Biol. Chem. 272, 21504–21508 (1997).
Little, K. D., Hemler, M. E. & Stipp, C. S. Dynamic regulation of a GPCR–tetraspanin–G protein complex on intact cells: central role of CD81 in facilitating GPR56–Gαq/11 association. Mol. Biol. Cell 15, 2375–2387 (2004).
Stacey, M. et al. The epidermal growth factor-like domains of the human EMR2 receptor mediate cell attachment through chondroitin sulfate glycosaminoglycans. Blood 102, 2916–2924 (2003).
Stacey, M., Lin, H. H., Hilyard, K. L., Gordon, S. & McKnight, A. J. Human epidermal growth factor (EGF) module-containing mucin-like hormone receptor 3 is a new member of the EGF-TM7 family that recognizes a ligand on human macrophages and activated neutrophils. J. Biol. Chem. 276, 18863–18870 (2001).
Stacey, M. et al. EMR4, a novel epidermal growth factor (EGF)-TM7 molecule up-regulated in activated mouse macrophages, binds to a putative cellular ligand on B lymphoma cell line A20. J. Biol. Chem. 277, 29283–29293 (2002).
Hamann, J., Vogel, B., van Schijndel, G. M. & van Lier, R. A. The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J. Exp. Med. 184, 1185–1189 (1996). This paper is the first to show that GPCRs can bind to ligands attached to the extracellular matrix of other cells, thereby further widening our concepts about the ligand preferences of GPCRs.
Hamann, J. et al. Characterization of the CD55 (DAF)-binding site on the seven-span transmembrane receptor CD97. Eur. J. Immunol. 28, 1701–1707 (1998).
Xu, L., Begum, S., Hearn, J. D. & Hynes, R. O. GPR56, an atypical G protein-coupled receptor, binds tissue transglutaminase, TG2, and inhibits melanoma tumor growth and metastasis. Proc. Natl Acad. Sci. USA 103, 9023–9028 (2006).
Liu, M. et al. GPR56, a novel secretin-like human G-protein-coupled receptor gene. Genomics 55, 296–305 (1999).
Sugita, S., Ichtchenko, K., Khvotchev, M. & Sudhof, T. C. α-Latrotoxin receptor CIRL/latrophilin 1 (CL1) defines an unusual family of ubiquitous G-protein-linked receptors. G-protein coupling not required for triggering exocytosis. J. Biol. Chem. 273, 32715–32724 (1998).
Krasnoperov, V., Bittner, M. A., Holz, R. W., Chepurny, O. & Petrenko, A. G. Structural requirements for α-latrotoxin binding and α-latrotoxin-stimulated secretion. A study with calcium-independent receptor of α-latrotoxin (CIRL) deletion mutants. J. Biol. Chem. 274, 3590–3596 (1999).
Hadjantonakis, A. K. et al. Celsr1, a neural-specific gene encoding an unusual seven-pass transmembrane receptor, maps to mouse chromosome 15 and human chromosome 22qter. Genomics 45, 97–104 (1997).
Nakayama, M. et al. Identification of high-molecular-weight proteins with multiple EGF-like motifs by motif-trap screening. Genomics 51, 27–34 (1998).
Usui, T. et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98, 585–595 (1999).
Curtin, J. A. et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr. Biol. 13, 1129–1133 (2003).
Nishimori, H. et al. A novel brain-specific p53-target gene, BAI1, containing thrombospondin type 1 repeats inhibits experimental angiogenesis. Oncogene 15, 2145–2150 (1997).
Osterhoff, C., Ivell, R. & Kirchhoff, C. Cloning of a human epididymis-specific mRNA, HE6, encoding a novel member of the seven transmembrane-domain receptor superfamily. DNA Cell Biol. 16, 379–389 (1997).
Shiratsuchi, T., Nishimori, H., Ichise, H., Nakamura, Y. & Tokino, T. Cloning and characterization of BAI2 and BAI3, novel genes homologous to brain-specific angiogenesis inhibitor 1 (BAI1). Cytogenet. Cell Genet. 79, 103–108 (1997).
Nikkila, H. et al. Sequence similarities between a novel putative G protein-coupled receptor and Na+/Ca2+ exchangers define a cation binding domain. Mol. Endocrinol. 14, 1351–1364 (2000).
Fredriksson, R., Lagerstrom, M. C., Hoglund, P. J. & Schioth, H. B. Novel human G protein-coupled receptors with long N-terminals containing GPS domains and Ser/Thr-rich regions. FEBS Lett. 531, 407–414 (2002).
Fredriksson, R., Gloriam, D. E., Hoglund, P. J., Lagerstrom, M. C. & Schioth, H. B. There exist at least 30 human G-protein-coupled receptors with long Ser/Thr-rich N-termini. Biochem. Biophys. Res. Commun. 301, 725–734 (2003).
Lopezjimenez, N. D. et al. Two novel genes, Gpr113, which encodes a family 2 G-protein-coupled receptor, and Trcg1, are selectively expressed in taste receptor cells. Genomics 85, 472–482 (2005).
Abe, J., Suzuki, H., Notoya, M., Yamamoto, T. & Hirose, S. Ig-hepta, a novel member of the G protein-coupled hepta-helical receptor (GPCR) family that has immunoglobulin-like repeats in a long N-terminal extracellular domain and defines a new subfamily of GPCRs. J. Biol. Chem. 274, 19957–19964 (1999).
Abe, J., Fukuzawa, T. & Hirose, S. Cleavage of Ig-Hepta at a “SEA” module and at a conserved G protein-coupled receptor proteolytic site. J. Biol. Chem. 277, 23391–23398 (2002).
Wreschner, D. H. et al. Generation of ligand-receptor alliances by “SEA” module-mediated cleavage of membrane-associated mucin proteins. Protein Sci. 11, 698–706 (2002).
McMillan, D. R., Kayes-Wandover, K. M., Richardson, J. A. & White, P. C. Very large G protein-coupled receptor-1, the largest known cell surface protein, is highly expressed in the developing central nervous system. J. Biol. Chem. 277, 785–792 (2002).
McMillan, D. R. & White, P. C. Loss of the transmembrane and cytoplasmic domains of the very large G-protein-coupled receptor-1 (VLGR1 or Mass1) causes audiogenic seizures in mice. Mol. Cell Neurosci. 26, 322–329 (2004).
Bjarnadottir, T. K. et al. Identification of novel splice variants of adhesion G protein-coupled receptors. Gene 387, 38–48 (2006).
Lagerstrom, M. C. et al. The evolutionary history and tissue mapping of GPR123: specific CNS expression pattern predominantly in thalamic nuclei and regions containing large pyramidal cells. J. Neurochem. 100, 1129–1142 (2007).
Bjarnadottir, T. K., Fredriksson, R. & Schioth, H. B. The gene repertoire and the common evolutionary history of glutamate, pheromone (V2R), taste(1) and other related G protein-coupled receptors. Gene 362, 70–84 (2005).
Kunishima, N. et al. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407, 971–977 (2000). This paper provides the first high-resolution structure of the extracellular ligand binding domain of a Glutamate family member, GRM1, from rat.
O'Hara, P. J. et al. The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron 11, 41–52 (1993).
Parmentier, M. L. et al. Conservation of the ligand recognition site of metabotropic glutamate receptors during evolution. Neuropharmacology 39, 1119–1131 (2000).
Muto, T., Tsuchiya, D., Morikawa, K. & Jingami, H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc. Natl Acad. Sci. USA 104, 3759–3764 (2007). This paper highlights the importance of the conserved linker region between the extracellular ligand binding domain and the transmembrane signalling region in Glutamate family receptors.
Brauner-Osborne, H., Jensen, A. A., Sheppard, P. O., O'Hara, P. & Krogsgaard-Larsen, P. The agonist-binding domain of the calcium-sensing receptor is located at the amino-terminal domain. J. Biol. Chem. 274, 18382–18386 (1999).
Brown, E. M. et al. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366, 575–580 (1993).
Kuang, D. et al. Molecular similarities in the ligand binding pockets of an odorant receptor and the metabotropic glutamate receptors. J. Biol. Chem. 278, 42551–42559 (2003).
Wellendorph, P. & Brauner-Osborne, H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene 335, 37–46 (2004).
Silve, C. et al. Delineating a Ca2+ binding pocket within the venus flytrap module of the human calcium-sensing receptor. J. Biol. Chem. 280, 37917–37923 (2005).
Hermans, E. & Challiss, R. A. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem. J. 359, 465–484 (2001).
Nakanishi, S. Molecular diversity of glutamate receptors and implications for brain function. Science 258, 597–603 (1992).
Kubo, Y., Miyashita, T. & Murata, Y. Structural basis for a Ca2+-sensing function of the metabotropic glutamate receptors. Science 279, 1722–1725 (1998).
Conigrave, A. D., Quinn, S. J. & Brown, E. M. L-Amino acid sensing by the extracellular Ca2+-sensing receptor. Proc. Natl Acad. Sci. USA 97, 4814–4819 (2000).
Xu, H. et al. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc. Natl Acad. Sci. USA 101, 14258–14263 (2004).
Rondard, P. et al. Coupling of agonist binding to effector domain activation in metabotropic glutamate-like receptors. J. Biol. Chem. 281, 24653–24661 (2006).
Yu, L. et al. NCD3G: a novel nine-cysteine domain in family 3 GPCRs. Trends Biochem. Sci. 29, 458–461 (2004).
Hu, J., Hauache, O. & Spiegel, A. M. Human Ca2+ receptor cysteine-rich domain. Analysis of function of mutant and chimeric receptors. J. Biol. Chem. 275, 16382–16389 (2000).
Tan, Y. M. et al. Autosomal dominant hypocalcemia: a novel activating mutation (E604K) in the cysteine-rich domain of the calcium-sensing receptor. J. Clin. Endocrinol. Metab. 88, 605–610 (2003).
Jiang, P. et al. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J. Biol. Chem. 279, 45068–45075 (2004).
Kaupmann, K. et al. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature 386, 239–246 (1997).
Martin, S. C., Russek, S. J. & Farb, D. H. Human GABABR genomic structure: evidence for splice variants in GABABR1 but not GABABR2. Gene 278, 63–79 (2001).
Galvez, T. et al. Mutagenesis and modeling of the GABAB receptor extracellular domain support a venus flytrap mechanism for ligand binding. J. Biol. Chem. 274, 13362–13369 (1999).
Reid, K. B. & Day, A. J. Structure–function relationships of the complement components. Immunol. Today 10, 177–180 (1989).
Ichinose, A., Bottenus, R. E. & Davie, E. W. Structure of transglutaminases. J. Biol. Chem. 265, 13411–13414 (1990).
Liu, J. et al. Molecular determinants involved in the allosteric control of agonist affinity in the GABAB receptor by the GABAB2 subunit. J. Biol. Chem. 279, 15824–15830 (2004).
Kniazeff, J. et al. Locking the dimeric GABAB G-protein-coupled receptor in its active state. J. Neurosci. 24, 370–377 (2004).
Shoback, D. M. et al. The calcimimetic cinacalcet normalizes serum calcium in subjects with primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 88, 5644–5649 (2003).
Malherbe, P. et al. Mutational analysis and molecular modeling of the binding pocket of the metabotropic glutamate 5 receptor negative modulator 2-methyl-6-(phenylethynyl)-pyridine. Mol. Pharmacol. 64, 823–832 (2003).
Litschig, S. et al. CPCCOEt, a noncompetitive metabotropic glutamate receptor 1 antagonist, inhibits receptor signaling without affecting glutamate binding. Mol. Pharmacol. 55, 453–461 (1999).
Gasparini, F., Kuhn, R. & Pin, J. P. Allosteric modulators of group I metabotropic glutamate receptors: novel subtype-selective ligands and therapeutic perspectives. Curr. Opin. Pharmacol. 2, 43–49 (2002).
Petrel, C. et al. Positive and negative allosteric modulators of the Ca2+-sensing receptor interact within overlapping but not identical binding sites in the transmembrane domain. J. Biol. Chem. 279, 18990–18997 (2004).
Petrel, C. et al. Modeling and mutagenesis of the binding site of Calhex 231, a novel negative allosteric modulator of the extracellular Ca2+-sensing receptor. J. Biol. Chem. 278, 49487–49494 (2003).
Knoflach, F. et al. Positive allosteric modulators of metabotropic glutamate 1 receptor: characterization, mechanism of action, and binding site. Proc. Natl Acad. Sci. USA 98, 13402–13407 (2001).
Jiang, P. et al. Identification of the cyclamate interaction site within the transmembrane domain of the human sweet taste receptor subunit T1R3. J. Biol. Chem. 280, 34296–34305 (2005).
Jiang, P. et al. Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J. Biol. Chem. 280, 15238–15246 (2005).
Binet, V. et al. The heptahelical domain of GABAB2 is activated directly by CGP7930, a positive allosteric modulator of the GABAB receptor. J. Biol. Chem. 279, 29085–29091 (2004).
Chen, Y. et al. Interaction of novel positive allosteric modulators of metabotropic glutamate receptor 5 with the negative allosteric antagonist site is required for potentiation of receptor responses. Mol. Pharmacol. 71, 1389–1398 (2007).
Wellendorph, P. et al. Deorphanization of GPRC6A: a promiscuous L-α-amino acid receptor with preference for basic amino acids. Mol. Pharmacol. 67, 589–597 (2005).
Christiansen, B., Hansen, K. B., Wellendorph, P. & Brauner-Osborne, H. Pharmacological characterization of mouse GPRC6A, an L-α--amino-acid receptor modulated by divalent cations. Br. J. Pharmacol. 150, 798–807 (2007).
Calver, A. R. et al. Molecular cloning and characterisation of a novel GABAB-related G-protein coupled receptor. Brain Res. Mol. Brain Res. 110, 305–317 (2003).
Cheng, Y. & Lotan, R. Molecular cloning and characterization of a novel retinoic acid-inducible gene that encodes a putative G protein-coupled receptor. J. Biol. Chem. 273, 35008–35015 (1998).
Brauner-Osborne, H. et al. Cloning and characterization of a human orphan family C G-protein coupled receptor GPRC5D. Biochim. Biophys. Acta 1518, 237–248 (2001).
Brauner-Osborne, H. & Krogsgaard-Larsen, P. Sequence and expression pattern of a novel human orphan G-protein-coupled receptor, GPRC5B, a family C receptor with a short amino-terminal domain. Genomics 65, 121–128 (2000).
Robbins, M. J. et al. Molecular cloning and characterization of two novel retinoic acid-inducible orphan G-protein-coupled receptors (GPRC5B and GPRC5C). Genomics 67, 8–18 (2000).
Ota, T. et al. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nature Genet. 36, 40–45 (2004).
Vinson, C. R., Conover, S. & Adler, P. N. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature 338, 263–264 (1989).
Wang, Y. et al. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J. Biol. Chem. 271, 4468–4476 (1996).
Wang, Y. K. et al. A novel human homologue of the Drosophila frizzled wnt receptor gene binds wingless protein and is in the Williams syndrome deletion at 7q11.23. Hum. Mol. Genet. 6, 465–472 (1997).
Zhao, Z., Lee, C. C., Baldini, A. & Caskey, C. T. A human homologue of the Drosophila polarity gene frizzled has been identified and mapped to 17q21.1. Genomics 27, 370–373 (1995).
Tokuhara, M., Hirai, M., Atomi, Y., Terada, M. & Katoh, M. Molecular cloning of human Frizzled-6. Biochem. Biophys. Res. Commun. 243, 622–627 (1998).
Sala, C. F., Formenti, E., Terstappen, G. C. & Caricasole, A. Identification, gene structure, and expression of human frizzled-3 (FZD3). Biochem. Biophys. Res. Commun. 273, 27–34 (2000).
Sagara, N., Toda, G., Hirai, M., Terada, M. & Katoh, M. Molecular cloning, differential expression, and chromosomal localization of human frizzled-1, frizzled-2, and frizzled-7. Biochem. Biophys. Res. Commun. 252, 117–122 (1998).
Saitoh, T., Hirai, M. & Katoh, M. Molecular cloning and characterization of human Frizzled-8 gene on chromosome 10p11.2. Int. J. Oncol. 18, 991–996 (2001).
Kirikoshi, H. et al. Molecular cloning and characterization of human Frizzled-4 on chromosome 11q14-q21. Biochem. Biophys. Res. Commun. 264, 955–961 (1999).
Koike, J. et al. Molecular cloning of Frizzled-10, a novel member of the Frizzled gene family. Biochem. Biophys. Res. Commun. 262, 39–43 (1999).
Bhanot, P. et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382, 225–230 (1996).
Murone, M., Rosenthal, A. & de Sauvage, F. J. Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr. Biol. 9, 76–84 (1999).
Slusarski, D. C., Corces, V. G. & Moon, R. T. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390, 410–413 (1997).
Riobo, N. A., Saucy, B., Dilizio, C. & Manning, D. R. Activation of heterotrimeric G proteins by Smoothened. Proc. Natl Acad. Sci. USA 103, 12607–12612 (2006).
Barnes, M. R., Duckworth, D. M. & Beeley, L. J. Frizzled proteins constitute a novel family of G protein-coupled receptors, most closely related to the secretin family. Trends Pharmacol. Sci. 19, 399–400 (1998).
Carron, C. et al. Frizzled receptor dimerization is sufficient to activate the Wnt/β-catenin pathway. J. Cell Sci. 116, 2541–2550 (2003).
Dann, C. E. et al. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature 412, 86–90 (2001). This paper provided the first high-resolution structure of the ligand binding region of a Frizzled family member.
Chen, C. M., Strapps, W., Tomlinson, A. & Struhl, G. Evidence that the cysteine-rich domain of Drosophila Frizzled family receptors is dispensable for transducing Wingless. Proc. Natl Acad. Sci. USA 101, 15961–15966 (2004).
Xu, Q. et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116, 883–895 (2004).
Robitaille, J. et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nature Genet. 32, 326–330 (2002).
Fritze, O. et al. Role of the conserved NPxxY(x)5, 6F motif in the rhodopsin ground state and during activation. Proc. Natl Acad. Sci. USA 100, 2290–2295 (2003).
Stone, D. M. et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 384, 129–134 (1996).
Nakano, Y. et al. Functional domains and sub-cellular distribution of the Hedgehog transducing protein Smoothened in Drosophila. Mech. Dev. 121, 507–518 (2004).
Chen, Y. & Struhl, G. In vivo evidence that Patched and Smoothened constitute distinct binding and transducing components of a Hedgehog receptor complex. Development 125, 4943–4948 (1998).
Taipale, J. et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 406, 1005–1009 (2000).
Chen, J. K., Taipale, J., Young, K. E., Maiti, T. & Beachy, P. A. Small molecule modulation of Smoothened activity. Proc. Natl Acad. Sci. USA 99, 14071–14076 (2002).
Frank-Kamenetsky, M. et al. Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J. Biol. 1, 10 (2002).
Nagayama, S. et al. Therapeutic potential of antibodies against FZD 10, a cell-surface protein, for synovial sarcomas. Oncogene 24, 6201–6212 (2005).
Shi, P., Zhang, J., Yang, H. & Zhang, Y. P. Adaptive diversification of bitter taste receptor genes in mammalian evolution. Mol. Biol. Evol. 20, 805–814 (2003).
Go, Y., Satta, Y., Takenaka, O. & Takahata, N. Lineage-specific loss of function of bitter taste receptor genes in humans and nonhuman primates. Genetics 170, 313–326 (2005).
Conte, C., Ebeling, M., Marcuz, A., Nef, P. & Andres-Barquin, P. J. Identification and characterization of human taste receptor genes belonging to the TAS2R family. Cytogenet. Genome Res. 98, 45–53 (2002).
Bufe, B., Hofmann, T., Krautwurst, D., Raguse, J. D. & Meyerhof, W. The human TAS2R16 receptor mediates bitter taste in response to β-glucopyranosides. Nature Genet. 32, 397–401 (2002).
Chandrashekar, J. et al. T2Rs function as bitter taste receptors. Cell 100, 703–711 (2000). This is one of the first reports to directly show that the bitter taste sensation is mediated through GPCRs.
Behrens, M. et al. The human taste receptor hTAS2R14 responds to a variety of different bitter compounds. Biochem. Biophys. Res. Commun. 319, 479–485 (2004).
Kuhn, C. et al. Bitter taste receptors for saccharin and acesulfame K. J. Neurosci. 24, 10260–10265 (2004).
Ueda, T. et al. Identification of coding single-nucleotide polymorphisms in human taste receptor genes involving bitter tasting. Biochem. Biophys. Res. Commun. 285, 147–151 (2001).
Pronin, A. N., Tang, H., Connor, J. & Keung, W. Identification of ligands for two human bitter T2R receptors. Chem. Senses 29, 583–593 (2004).
Gloriam, D. E. et al. The repertoire of trace amine G-protein-coupled receptors: large expansion in zebrafish. Mol. Phylogenet Evol. 35, 470–482 (2005).
Kostenis, E. A glance at G-protein-coupled receptors for lipid mediators: a growing receptor family with remarkably diverse ligands. Pharmacol. Ther. 102, 243–257 (2004).
Cavalier-Smith, T. Only six kingdoms of life. Proc. Biol. Sci. 271, 1251–1262 (2004).
Pupillo, M., Insall, R., Pitt, G. S. & Devreotes, P. N. Multiple cyclic AMP receptors are linked to adenylyl cyclase in Dictyostelium. Mol. Biol. Cell 3, 1229–1234 (1992).
Blumer, K. J. & Thorner, J. β and γ subunits of a yeast guanine nucleotide-binding protein are not essential for membrane association of the α subunit but are required for receptor coupling. Proc. Natl Acad. Sci. USA 87, 4363–4367 (1990).
Xue, Y., Batlle, M. & Hirsch, J. P. GPR1 encodes a putative G protein-coupled receptor that associates with the Gpa2p Gα subunit and functions in a Ras-independent pathway. Embo J. 17, 1996–2007 (1998).
Prabhu, Y. & Eichinger, L. The Dictyostelium repertoire of seven transmembrane domain receptors. Eur. J. Cell Biol. 85, 937–946 (2006).
Williams, J. G., Noegel, A. A. & Eichinger, L. Manifestations of multicellularity: Dictyostelium reports in. Trends Genet. 21, 392–398 (2005).
Perez, D. M. From plants to man: the GPCR “tree of life”. Mol. Pharmacol. 67, 1383–1384 (2005).
Perez, D. M. The evolutionarily triumphant G-protein-coupled receptor. Mol. Pharmacol. 63, 1202–1205 (2003).
Parameswaran, N. & Spielman, W. S. RAMPs: the past, present and future. Trends Biochem. Sci. 31, 631–638 (2006).
Gurevich, E. V. & Gurevich, V. V. Arrestins: ubiquitous regulators of cellular signaling pathways. Genome Biol. 7, 236 (2006).
McCudden, C. R., Hains, M. D., Kimple, R. J., Siderovski, D. P. & Willard, F. S. G-protein signaling: back to the future. Cell. Mol. Life Sci. 62, 551–577 (2005).
Whitehead, I. P., Zohn, I. E. & Der, C. J. Rho GTPase-dependent transformation by G protein-coupled receptors. Oncogene 20, 1547–1555 (2001).
Shi, P. & Zhang, J. Contrasting modes of evolution between vertebrate sweet/umami receptor genes and bitter receptor genes. Mol. Biol. Evol. 23, 292–300 (2005).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Donnelly, D. The arrangement of the transmembrane helices in the secretin receptor family of G-protein-coupled receptors. FEBS Lett. 409, 431–436 (1997).
Couvineau, A. et al. Highly conserved aspartate 68, tryptophane 73 and glycine 109 in the N-terminal extracellular domain of the human VIP receptor are essential for its ability to bind VIP. Biochem. Biophys. Res. Commun. 206, 246–252 (1995).
Carruthers, C. J., Unson, C. G., Kim, H. N. & Sakmar, T. P. Synthesis and expression of a gene for the rat glucagon receptor. Replacement of an aspartic acid in the extracellular domain prevents glucagon binding. J. Biol. Chem. 269, 29321–29328 (1994).
Holtmann, M. H., Ganguli, S., Hadac, E. M., Dolu, V. & Miller, L. J. Multiple extracellular loop domains contribute critical determinants for agonist binding and activation of the secretin receptor. J. Biol. Chem. 271, 14944–14949 (1996).
Bisello, A. et al. Parathyroid hormone-receptor interactions identified directly by photocross-linking and molecular modeling studies. J. Biol. Chem. 273, 22498–22505 (1998).
Di Paolo, E. et al. Role of charged amino acids conserved in the vasoactive intestinal polypeptide/secretin family of receptors on the secretin receptor functionality. Peptides 20, 1187–1193 (1999).
Zhou, A. T. et al. Direct mapping of an agonist-binding domain within the parathyroid hormone/parathyroid hormone-related protein receptor by photoaffinity crosslinking. Proc. Natl Acad. Sci. USA 94, 3644–3649 (1997).
Sydow, S., Flaccus, A., Fischer, A. & Spiess, J. The role of the fourth extracellular domain of the rat corticotropin-releasing factor receptor type 1 in ligand binding. Eur. J. Biochem. 259, 55–62 (1999).
Piserchio, A., Bisello, A., Rosenblatt, M., Chorev, M. & Mierke, D. F. Characterization of parathyroid hormone/receptor interactions: structure of the first extracellular loop. Biochemistry 39, 8153–8160 (2000).
Solano, R. M. et al. Two basic residues of the h-VPAC1 receptor second transmembrane helix are essential for ligand binding and signal transduction. J. Biol. Chem. 276, 1084–1088 (2001).
Runge, S. et al. Three distinct epitopes on the extracellular face of the glucagon receptor determine specificity for the glucagon amino terminus. J. Biol. Chem. 278, 28005–28010 (2003).
Lins, L. et al. The human VPAC1 receptor: three-dimensional model and mutagenesis of the N-terminal domain. J. Biol. Chem. 276, 10153–10160 (2001).
Langer, I., Vertongen, P., Perret, J., Waelbroeck, M. & Robberecht, P. Lysine 195 and aspartate 196 in the first extracellular loop of the VPAC1 receptor are essential for high affinity binding of agonists but not of antagonists. Neuropharmacology 44, 125–131 (2003).
Marchler-Bauer, A. et al. CDD: a Conserved Domain Database for protein classification. Nucleic Acids Res. 33, D192–D196 (2005).
Lin, H. H., Stacey, M., Hamann, J., Gordon, S. & McKnight, A. J. Human EMR2, a novel EGF-TM7 molecule on chromosome 19p13.1, is closely related to CD97. Genomics 67, 188–200 (2000).
Zhang, Z. et al. Three adjacent serines in the extracellular domains of the CaR are required for L-amino acid-mediated potentiation of receptor function. J. Biol. Chem. 277, 33727–33735 (2002).
Galvez, T. et al. Mapping the agonist-binding site of GABAB type 1 subunit sheds light on the activation process of GABAB receptors. J. Biol. Chem. 275, 41166–41174 (2000).
Laurie, D. J., Schoeffter, P., Wiederhold, K. H. & Sommer, B. Cloning, distribution and functional expression of the human mGlu6 metabotropic glutamate receptor. Neuropharmacology 36, 145–152 (1997).
Hendy, G. N., D'Souza-Li, L., Yang, B., Canaff, L. & Cole, D. E. Mutations of the calcium-sensing receptor (CASR) in familial hypocalciuric hypercalcemia, neonatal severe hyperparathyroidism, and autosomal dominant hypocalcemia. Hum. Mutat. 16, 281–296 (2000).
Fatkenheuer G. et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nature Med. 11, 1170–1172 (2005).
Acknowledgements
We thank J.-P. Pin, R. Bywater, D. Rognan, K. Kullander and C. Pickering for valuable comments on the manuscript. We also thank R. Fredriksson for fruitful discussions on the topic. This study was supported by The Swedish Research Council and the Swedish Brain Foundation.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Phylogenetic classification
-
The classification of protein sequences that is based on primary sequence similarity.
- Sequence hidden Markov model
-
A statistical representation of a multiple sequence alignment. The model can later be used as a search tool to identify similar sequences.
- Bilateral
-
The body of a bilateral animal is symmetrical around the midline, which results in two almost mirror image halves. The Bilateria can be divided into two main groups: deuterostomes and proto-stomes, in which mammals belong to the former.
- Pharmacophore
-
The steric and electronic features of a ligand that are necessary to ensure optimal interactions with a biological target structure and to trigger (or to block) its biological response.
- Allosteric ligands
-
Ligands binding to a site that is separate from the site of the endogenous ligand/ligands.
Rights and permissions
About this article
Cite this article
Lagerström, M., Schiöth, H. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov 7, 339–357 (2008). https://doi.org/10.1038/nrd2518
Issue Date:
DOI: https://doi.org/10.1038/nrd2518