Abstract
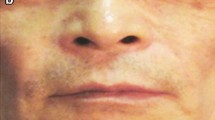
The search for an ideal filler for soft tissue augmentation still continues. Because aging changes are continuous, temporary fillers should be preferred against permanent ones. Since 1999, the poly-L-lactic acid filler (PLA) has been marketed in Europe as Newfill. As a synthetic biocompatible polymer, PLA originally was used in suture materials and screws. In 2004, the U.S. Food and Drug Administration approved PLA under the name of Sculptra for the treatment of human immunodeficiency virus–related facial lipoatrophy. This study aimed to evaluate a 3-year follow-up investigation into the effect of PLA implant injection for the treatment of sunken nasolabial folds. Between October 2003 and February 2004, 10 women with a median age of 54 years (range, 43–60 years) were injected with polylactic acid hydrogel (Newfill) in the nasolabial fold area for aesthetic reasons. All the patients underwent three injections: one injection per month for 3 months. Evaluation of the results based on clinical examination and photography was performed at each session, at 6 months, and then 36 months after the third session. Injectable PLA was able to correct nasolabial folds successfully with a more lasting result than absorbable fillers commonly used in clinical practice, such as hyaluronic acid and collagen. Careful and standardized photographic documentation is indispensable.



Similar content being viewed by others
References
Bauer U, Vleggaar D (2006) Response to Newfill injections may induce late-onset foreign body granulomatous reaction. Plast Reconstr Surg 118:265
Fagien S (2000) Facial soft tissue augmentation with injectable autologous and allogeneic human tissue collagen matrix (Autologen and Dermalogen). Plast Reconstr Surg 105:362
Jansen DA, Graivier MH (2003) Soft tissue substitutes in perioral augmentation. Semin Plast Surg 17
Laglenne S (2000) Un noveau produit de comblement des rides, entirerment resorbable. Dermatologie 54:30–33
Lam S, Azizzadeh B, Graivier M (2006) Injectable poly-L-lactic acid (Sculptra): technical consideratios in soft tissue contouring. Plast Reconstr Surg 118(Suppl):55S–63S
Lemperle G, Holmes RE, Cohen SR, Lemperle SM (2001) A classification of facial wrinkles. Plast Reconstr Surg 108:1736
Lemperle F, Morhenn V, Charrier U (2003) Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesth Plast Surg 27:354
Lemperle G, Morhenn VB, Pestonjamasp V, Gallo RL (2004) Migration studies and histology of injectable microspheres of different sizes in mice. Plast Reconstr Surg 113:1380
Perry C (2004) Poly-L-lactic acid. Am J Clin Dermatol 5:361–366
Rohrich RJ, Rios JL, Fagien S (2003) Role of new fillers in facial rejuvenation: a cautious outlook. Plast Reconstr Surg 112
Valantin MA, Aubron-Olivier C, Ghosn J, Laglenne E, Pauchard M, Schoena H, Bousqueta R, Katz P, Costagliola D, Katlama C (2003) Polylactic acid implants (Newfill) to correct facial lipoatrophy in HIV-infected patients: results of the open label study VEGA. AIDS 17:2471–2477
Vleggaar D (2005) Facial volumetric correction with injectable poly-L-lactic acid. Dermatol Surg 31:1511–1518
Vleggaar D (2006) Soft tissue augmentation and the role of poly-L-lactic acid. Plast Reconstr Surg 118(Suppl.):46S
Vleggaar D, Bauer D (2004) Facial enhancement and the European experience with Sculptra (poly-L-lactic acid). J Drugs Dermatol 3:542–547
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salles, A.G., Lotierzo, P.H., Gimenez, R. et al. Evaluation of the Poly-L-Lactic Acid Implant for Treatment of the Nasolabial Fold: 3-Year Follow-Up Evaluation. Aesth Plast Surg 32, 753–756 (2008). https://doi.org/10.1007/s00266-008-9182-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-008-9182-2