Abstract
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, enhances synaptic transmission and regulates neuronal proliferation and survival. Functional interactions between adenosine A2A receptors (A2ARs) and BDNF have been recently reported. In this article, we report some recent findings from our group showing that A2ARs regulate both BDNF functions and levels in the brain. Whereas BDNF (10 ng/ml) increased the slope of excitatory postsynaptic field potentials (fEPSPs) in hippocampal slices from wild-type (WT) mice, it was completely ineffective in slices taken from A2AR knock-out (KO) mice. Furthermore, enzyme immunoassay studies showed a significant reduction in hippocampal BDNF levels in A2AR KO vs. WT mice. Having found an even marked reduction in the striatum of A2AR KO mice, and as both BDNF and A2ARs have been implicated in the pathogenesis of Huntington’s disease (HD), an inherited striatal neurodegenerative disease, we then evaluated whether the pharmacological blockade of A2ARs could influence striatal levels of BDNF in an experimental model of HD-like striatal degeneration (quinolinic acid-lesioned rats) and in a transgenic mice model of HD (R6/2 mice). In both QA-lesioned rats and early symptomatic R6/2 mice (8 weeks), the systemic administration of the A2AR antagonist SCH58261 significantly reduced striatal BDNF levels. These results indicate that the presence and the tonic activation of A2ARs are necessary to allow BDNF-induced potentiation of synaptic transmission and to sustain a normal BDNF tone. The possible functional consequences of reducing striatal BDNF levels in HD models need further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain-derived neurotrophic factor (BDNF) is an endogenous glycoprotein belonging to neurotrophins, a family of signalling molecules that play a key role in regulating neuronal proliferation, differentiation and survival [1–3]. Among the neurotrophins, BDNF has the widest distribution in the central nervous system [4, 5]. In the adult hippocampus, BDNF is critically involved in the regulation of synaptic plasticity [6] and facilitates long-term potentiation (LTP) [7–10; see 11 for review]. Most biological effects of BDNF are mediated by the tyrosine kinase receptor TrkB. Both BDNF and its receptors are highly expressed in the hippocampus, and the activation state of the complex BDNF/TrkB appears critical for modulating synaptic efficacy [12] and the response to excitotoxic injury [13, 14].
In hippocampal neurones, adenosine has been reported to transactivate TrkB receptors, an effect involving the A2A receptor subtype (A2ARs) [15]. Furthermore, Diogenes et al. [16] reported that A2ARs facilitate the excitatory action of BDNF on hippocampal synaptic transmission.
Besides its involvement in hippocampal functions, BDNF is also very important for the survival of striatal neurons and the activity of corticostriatal synapses [17]. Impairment in BDNF function is though to play a major role in Huntington’s disease (HD), an inherited neurodegenerative disease caused by a mutation in the protein huntingtin and characterised by marked striatal degeneration (see [18] for review). Specifically, it has been shown that the activity of BDNF depends on the presence of normal huntingtin [19–21]. In vitro and in vivo data showed that wild-type huntingtin, but not the mutant protein, stimulates cortical BDNF production by acting at the level of Bdnf gene transcription [20, 21]. At the corticostriatal synapse, BDNF controls glutamate release and its exogenous administration allows striatal neurons to survive excitotoxin-induced neurodegeneration [22].
Mounting evidence also indicates an involvement of striatal A2A receptors in HD (see [23] and [24] for reviews). First, A2ARs are mainly localised on the neurons that degenerate early in HD [25]. Second, A2ARs and underlying signalling systems undergo profound changes in cellular and animal models of HD (see [24] for review). Thus, not only A2ARs seem to regulate BDNF functions in the brain, but both BDNF and A2ARs seem to be implicated in HD.
In this article we report and critically discuss some recent findings on the following issues obtained by our group: (1) the regulatory role exerted by hippocampal A2ARs on BDNF functions and levels as revealed by studies performed in A2AR KO mice, and (2) the effects of A2AR blockade on striatal BDNF levels in experimental models of HD or HD-like striatal degeneration.
In hippocampal slices from wild-type mice, the facilitatory effects exerted by BDNF on synaptic transmission require the endogenous activation of A2AR
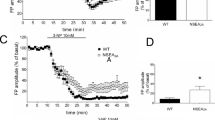
In hippocampal slices from wild-type (WT) mice (400-μm thick, see [26] for a detailed methodological description), BDNF (10 ng/ml over 30 min) induced a long-lasting increase in the slope of field excitatory postsynaptic potential (fEPSP) recorded in the CA1 area (Fig. 1). This effect was abolished not only by the inhibitor of Trk phosphorylation K-252a, but also by the selective adenosine A2A antagonists ZM241385 and SCH58261 (Tebano et al., manuscript in preparation). The effects of A2ARs seem to involve the cyclic adenosine monophosphate/protein kinase A (cAMP-PKA) pathway, the main transduction mechanism operated by A2ARs, as the selective PKA inhibitor KT 5720 abolished the excitatory action of BDNF (Tebano et al., manuscript in preparation).
Brain-derived neurotrophic factor (BDNF) facilitates synaptic transmission in hippocampal slices from wild-type (WT) but not A2A receptor (R) knock-out (KO) mice. In hippocampal slices from WT mice, BDNF (10 ng/ml) induces an increase of the excitatory postsynaptic field potentials (fEPSP) slope, whereas a lower concentration (5 ng/ml) is ineffective. BDNF (5, 10 and 20 ng/ml) was totally ineffective in hippocampal slices from A2AR KO mice. *P < 0.05 vs. baseline (paired t test)
The finding that BDNF enhances synaptic transmission in mice hippocampal slices confirms and strengthens previous data on the acute synaptic effects of BDNF at adult central synapses [17, 27]. Although the mechanisms responsible for such synaptic effects are not completely understood, they do not seem to be related to the neuroprotective ability of BDNF. That the above functions of BDNF recognise different molecular mechanisms is indicated by the fact that ligand-induced TrB translocation into lipid rafts is required for short-term synaptic modulation, but not neuronal survival, by BDNF [28]. Whatever the mechanisms, the blocking effect of ZM241385 and SCH58261 suggests that A2ARs have to be tonically activated by endogenous adenosine to manifest BDNF effects. To further explore the apparently “permissive” role of adenosine A2ARs on the synaptic effects of BDNF, A2AR KO mice were used.
BDNF is no longer able to facilitate synaptic transmission in hippocampal slices from A2A KO mice
A2AR KO mice (A2AR-/-) were generated, as previously described [29]. In hippocampal slices from A2AR KO mice, BDNF was no longer able to increase the fEPSP slope (Tebano et al., manuscript in preparation). As shown in Fig. 1, none of the tested concentrations of BDNF (5, 10 and 20 ng/ml) potentiated the synaptic response in slices from A2AR KO mice. Since the effectiveness of BDNF may depend on the proper expression of its receptors, we compared the levels of TrkB protein in WT and A2A KO mice by Western blotting. By using primary anti-TrkB antibody (Bioscience), we found that the levels of full-length TrkB isoforms were very similar in hippocampal extracts from WT and A2AR KO mice (Tebano et al., manuscript in preparation). Thus, the loss of BDNF activity observed in A2AR KO mice is not associated with a reduced density of TrkB receptors.
BDNF protein levels are significantly reduced in the brain of A2AR KO vs. WT mice
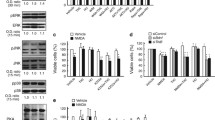
The BDNF protein levels were then measured on hippocampal extracts from A2AR KO and WT mice using the BDNF immunoassay system [enzyme-linked immunosorbent assay (ELISA) kit; Promega]. Interestingly, hippocampal BDNF levels were significantly lower in the A2AR KO mice compared with the WT littermates. As shown in Fig. 2, such an effect was not confined to the hippocampus, as a significant loss of BDNF levels was observed also in the striatum of A2A KO vs. WT mice.
Brain-derived neurotrophic factor (BDNF) levels are significantly reduced in the hippocampus and striatum of A2A receptor (R) knock-out (KO) mice. BDNF concentration was significantly reduced in extracts of hippocampal and striatal tissues prepared from A2AR mice compared with wild-type WT mice. The amount of BDNF (total) is expressed as pg/mg of wet tissue [means ± standard error of the mean (SEM) from five mice/group]. *P < 0.05 vs. WT
This result indicates that the presence of A2ARs is required to maintain normal BDNF levels in the brain. Although the mechanism by which A2ARs regulate BDNF is yet undetermined, it is worth mentioning that the cAMP-PKA pathway (the main transduction system operated by A2ARs) has been implicated not only in “gating” BDNF functions [30], but also in modulating BDNF gene transcription [31] and release [32]. Furthermore, Kolarow et al. [33] showed that the PKA inhibitor Rp-cAMP significantly inhibited and delayed BDNF secretion in hippocampal neurons. Interestingly, the endogenous state of activation of A2ARs seems to be adequate to sustain a normal BDNF tone in WT mice, as no increase in the hippocampal levels of BDNF were observed in WT mice treated with a single (0.5 mg/kg i.p sacrifice 24 h later) or with repeated (0.5 mg/kg per day over 5 days) doses of the A2A agonist CGS 21680.
On their whole, the experiments performed in A2A KO mice indicate that A2ARs are essential for the tone and synaptic activity of BDNF. It should be noted, however, that the stimulation or the blockade of A2ARs can induce effects very different from those achieved after stimulation or blockade of the BDNF/TRkB system. For instance, whereas BDNF is thought to play an important role in memory processes [34, 35], adenosine A2ARs seem to negatively influence them. Indeed, an improvement in spatial memory has been reported in A2AR KO mice [36], whereas working memory was impaired in rats overexpressing A2ARs [37]. Another consideration that apparently weakens the importance of the A2AR/BDNF interaction is that A2ARs and TrkB Rs undergo opposite age-related changes in the hippocampus [38]. According to the authors’ interpretation, however, the relationship between age-related changes in the density of TrkB and A2AR receptors does play a role in the modulation of BDNF effects, thus even reinforcing the hypothesis of a close functional interplay between A2ARs and BDNF.
A2ARs regulate striatal BDNF levels in experimental models of HD or HD-like striatal degeneration
Two different models of HD were used: the quinolinic-acid (QA)-lesioned rats (pathogenetic model of HD-like striatal degeneration, [39]) and transgenic R6/2 mice (genetic HD model, [40]). For the method of QA-induced striatal lesions, see [41]. Briefly, QA (180 nmol/1 μl) was unilaterally injected in the striatum of anaesthetised rats, whereas vehicle [1 μl phosphate-buffered saline (PBS)] was injected in the other side. SCH58261 was administered at the doses of 0.01 and 1 mg/kg i.p. 20 min before QA lesion. The levels of BDNF were measured 24 h after surgery. As shown in Fig. 3, BDNF levels were significantly increased in the QA-lesioned vs. the control side. In animals pretreated with both doses of SCH58261 the rise in striatal BDNF was completely prevented. Worthy of note, SCH58261 markedly reduced BDNF levels in the control (vehicle-injected) side also (Fig. 3). As the early rise in BDNF levels most probably reflects a compensatory mechanism following QA injection, one can consider that SCH58261 may worsen QA effects by preventing BDNF increase. Previous studies have, however, demonstrated that this is not the case. We found, indeed, that whereas SCH58261 exerted very clear neuroprotective effects towards QA at 0.01 mg/kg i.p., it was no longer neuroprotective when administered at 1 mg/kg [39]. The finding that SCH58261 reduces BDNF levels irrespective of the dose (neuroprotective or non-neuroprotective) indicates that early changes in the striatal BDNF levels do not seem to correlate with the clinical and neuropathological outcome in the QA model.
The A2A receptor (R)-antagonist SCH58261 reduces striatal brain-derived neurotrophic factor (BDNF) levels in control and quinolinic-acid (QA)-lesioned rats. QA (180 nmol/1 μl) was unilaterally injected in the striatum of anaesthetised rats, whereas vehicle [1 μl phosphate-buffered saline (PBS)] was injected in the other side. SCH58261 was administered at the doses of 0.01 and 1 mg/kg i.p., 20 min before QA lesion. The levels of free mature BDNF were measured 24 h after surgery. BDNF levels were significantly increased in the QA-lesioned vs. the control side. In animals pretreated with both doses of SCH58261, the rise in striatal BDNF was completely prevented. N 5/group. °P < 0.05 vs. PBS. *P < 0.05 vs. corresponding vehicle
R6/2 HD mice may represent a more suitable model to evaluate whether A2AR antagonists have a worsening influence through the reduction of striatal BDNF levels. Indeed, the administration of BDNF has been reported to have beneficial effects in transgenic models of HD [42, 43]. Considering that in the transgenic mice the neurodegenerative process progressively takes place over several weeks, to ascertain whether A2AR blockade could influence striatal BDNF levels, we decided to treat R6/2 mice chronically with SCH58261. The drug was administered at the dose of 0.01 mg/kg per day i.p. over 1 or 3 weeks starting from the fifth week of age. Another group of R6/2 mice was treated with vehicle over 3 weeks. BDNF levels were then assayed at the beginning of the symptomatic phase (8 weeks of age). Three groups of age-matched WT littermates were used as controls and treated in the same way described as above. As shown in Fig. 4, BDNF protein levels were not reduced, and showed instead a nonsignificant tendency to increase, in the striatum of early symptomatic R6/2 vs. WT mice. This finding was unexpected, as a significant decrease in BDNF mRNA has been recently reported in the striatum of R6/2 mice of the same age [43]. Although we have not explored the mechanisms responsible for such a discrepancy, a dissociation between the levels of BDNF protein and mRNA has already been observed in the brains of transgenic R6/1 HD mice [44]. Another reason for which one would expect a reduction in BDNF levels in the striatum of R6/2 mice is that a marked reduction in the expression of A2ARs (which, as mentioned above, are very important to maintain normal BDNF levels) was reported in these mice [45]. More recently, however, the A2AR-stimulated adenylyl cyclase, the A2AR density, and their affinity for the agonist CGS21680 were found unchanged in the striatum of clearly symptomatic R6/2 mice [46].
The A2A receptor (R)-antagonist SCH58261 reduces striatal brain-derived neurotrophic factor (BDNF) levels in R6/2 HD mice SCH58261 was administered at the dose of 0.01 mg/kg per day i.p. over 1 or 3 weeks starting from the fifth week of age. Free mature BDNF levels were then assayed at the beginning of the symptomatic phase (8 weeks of age). Three groups of age-matched wild-type (WT) littermates were used as controls and treated in the same way as described above. Both schedules (1 and 3 weeks) of SCH treatment significantly reduced BDNF levels in the striatum of either R6/2 and WT mice. N 4–7/group. *P < 0.05 vs. corresponding control group (two-tailed unpaired t test)
As for the influence of A2AR blockade, both schedules (1 and 3 weeks) of SCH treatment significantly reduced BDNF levels in the striatum of either R6/2 and WT mice (Fig. 4). Again, however, such an effect does not necessarily imply a negative influence of SCH58261 on HD mice. Indeed, in recent experiments, we found that SCH treatment (0.01 mg/kg per day i.p. between the 5th and the 6th weeks of age) exerted some beneficial effects, namely, normalisation of emotional behaviour and restoration of a normal sensitivity to NMDA in corticostriatal slices (Domenici et al., manuscript in preparation). Thus, although the pharmacological blockade of A2ARs significantly reduces striatal BDNF levels in experimental models of HD or HD-like neurodegeneration, this does not seem to have a negative impact on the course of the disease. Of course, it is still possible that reducing BDNF levels at other time points of the pathological process has a much marked influence on the disease. This issue, as well as the evaluation of BDNF protein changes at different stages of the disease, will be the object of future studies.
Conclusions
Over the last several years, BDNF has emerged not only as a potent neuromodulator but also as a substance exerting fast excitatory actions in neurons, controlling resting membrane potential, neuronal excitability and synaptic transmission and participating in the induction of long-term changes in synaptic transmission [47]. In agreement with the recent evidence that A2ARs play a major role in regulating BDNF functions [16, 48], data reported in this article indicate that the presence and tonic activation of A2ARs are necessary to allow BDNF-induced potentiation of synaptic transmission. As for the mechanisms responsible for the permissive effects of A2ARs, data indicate an involvement of the cAMP-PKA pathway, the main transduction mechanism operated by A2ARs. The finding of reduced BDNF levels in the brain of A2AR KO mice indicates that A2ARs are critical not only to allow the synaptic effects of BDNF but also to maintain normal BDNF levels. Findings also point out that A2ARs tonically regulate BDNF levels in different areas of the brain, irrespective of their levels of expression. The tonic effect exerted by A2ARs in the regulation of BDNF levels is also evident in experimental models of HD (R6/2 mice) and of HD-like striatal degeneration (QA lesion in rats).
Given that BDNF delivery has beneficial effects in models of HD [42, 43], the possible functional consequences of reducing striatal BDNF levels in those models need to be clarified. In particular, further investigations on trophic factors and associated signal pathways are warranted before A2AR antagonists can be considered as a suitable therapeutic approach to HD [24].
References
Vicario-Abejón C, Owens D, McKay R et al (2002) Role of neurotrophins in central synapse formation and stabilization. Nat Rev Neurosci 3:965–974
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736
Kalb R (2005) The protean actions of neurotrophins and their receptors on the life and death of neurons. Trends in Neurosciences 28:5–11
Schmidt-Kastner R, Wetmore C, Olson L (1996) Comparative study of brain-derived neurotrophic factor messenger RNA and protein at cellular level suggests multiple roles in hippocampus, striatum and cortex. Neuroscience 74:161–183
Conner JM, Lauterborn JC, Yan Q et al (1997) Distribution of brain derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci 17:2295–2313
Lu B (2003) BDNF and activity-dependent synaptic modulation. Learn Mem 10:86–98
Figurov A, Pozzo-Miller LD, Olafsson P et al (1996) Regulation of synaptic responses to high frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381:706–709
Korte M, Carroll P, Wolf E et al (1995) Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA 92:8856–8860
Korte M, Griesbeck O, Gravel C et al (1996) Virus-mediated gene transfer into hippocampal CA1 region restores long term potentiation in brain derived neurotrophic factor mutant mice. Proc Natl Acad Sci USA 93:12547–12552
Patterson SL, Abel T, Deuel TA et al (1996) Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16:1137–1145
Bramham CR, Messaoudi E (2005) BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol 76:99–125
Nagappan G, Lu B (2005) Activity-dependent modulation of the BDNF receptor TrkB: mechanisms and implications. Trends Neurosc 28:464–471
Canals J.M, Checa N, Marco S et al (1999) The neurotrophin receptors trkA, trkB and trkC are differentially regulated after excitotoxic lesion in rat striatum. Brain Res Mol Brain Res 69:242–248
Checa N, Canals JM, Gratacos E et al (2001) TrkB and TrkC are differentially regulated by excitotoxicity during development of the basal ganglia. Exp Neurol 172:282–292
Lee FS, Chao MV (2001) Activation of trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci USA 98:3555–3560
Diogenes MJ, Fernandes CC, Sebastião AM et al (2004) Activation of adenosine A2A receptor facilitates brain-derived neurotrophic factor modulation of synaptic transmission in hippocampal slices. J Neurosci 24:2905–2913
Cattaneo E, Zuccato C, Tartari M et al (2005) Normal Huntingtin function: an alternative approach to Huntington’s disease. Nat Rev Neurosci 6:919–930
Zuccato C, Cattaneo E (2007) Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol 81:294–330
Hodgson, JG, Agopyran, N, Gutekunst CA et al (1999) A YAC mouse model for Huntington’s disease with full-length mutant Huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron 23:181–192
Zuccato C, Ciammola A, Rigamonti D et al (2001) Loss of Huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science 293:493–498
Zuccato C, Tartari M, Crotti A et al (2003) Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet 35:76–83
Bemelmans AP, Horellou P, Pradier L et al (1999) Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington’s disease, as demonstrated by adenoviral gene transfer. Hum Gene Ther 10:2987–2997
Blum D, Hourez R, Galas MC et al (2003) Adenosine receptors and Huntington’s disease: implications for pathogenesis and therapeutics. Lancet Neurol 2: 366–374
Popoli P, Blum D, Martire A et al. (2007) Functions, dysfunctions and therapeutic potential of adenosine A2A receptors in Huntington’s disease. Prog Neurobiol 81(5–6):331–348
Glass M, Dragunow M, Faull RLM (2000) The pattern of neurodegeneration in Huntington’s disease: a comparative study of cannabinoid, dopamine adenosine and GABAA receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience 97:505–519
Tebano MT, Martire A, Rebola N et al (2005) Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: a possible key mechanism in the modulation of N-methyl-D-aspartate effects. J Neurochem 95:1188–1200
Poo M (2001) Neurotrophins as synaptic modulators. Nature 2:24–32
Suzuki S, Numakawa T, Shimazu K et al (2004) BDNF-induced recruitment of TrkB receptor into neuronal lipid rafrs: roles in synaptic modulation. J Cell Biol 167:1205–1215
Chen JF, Huang Z, Ma J, Zhu J et al (1999) A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci19:9192–9200
Boulanger L, Poo MM (1999) Gating of BDNF-induced synaptic potentiation by cAMP. Science 284:1982–1984
Fang H, Chartier J, Sodja C et al (2003) Transcriptional activation of the human brain-derived neurotrophic factor gene promoter III by dopamine signalling in NT2/N neurons. J Biol Chem 278:26401–26409
Patterson SL, Pittenger C, Morozov A et al(2001) Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phosphoMAP kinase. Neuron 32:123–140
Kolarow R, Brigadski T, Lessmann V (2006) Intracellular targeting of neurotrophins and signalling cascades involved in synaptic secretion of BDNF and NT3. Society for Neurosci, Annual Meeting, Atlanta USA, 14–18 October 2006
Tyler WJ, Alonso M, Bramhan CR, Pozzo-Miller LD (2002) From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem 9:224–237
Heldt SA, Stanek L, Chatwal JP, Ressler KJ (2007) Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry 12:656–670
Wang JH, Ma YY, van den Buuse M (2006) Improved spatial recognition memory in mice lacking adenosine A2A receptors. Exp. Neurol 199:438–445
Gimenez-Llort L, Schiffmann SN, Shmidt T et al (2007). Working memory deficits in transgenic rats overexpressing human adenosine A2A receptors in the brain. Neurobiol Learn Mem 87:42–56
Diogenes JM, Assaife-Lopes N, Pinto-Duarte A, Ribeiro JA, Sebastiao A (2007) Influence of age on BDNF modulation of hippocampal synaptic transmission: interplay with adenosine A2A receptors. Hippocampus 17:577–585
Popoli P, Pintor A, Domenici MR et al (2002) Blockade of striatal adenosine A2A receptor reduces, through a presynaptic mechanism, quinolinic acid-induced excitotoxicity: possible relevance to neuroprotective interventions in neurodegenerative diseases of the striatum. J Neurosci 22:1967–1975
Mangiarini L, Sathasivam K, Seller M et al (1996) Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87:493–506
Minghetti L, Greco A, Potenza RL et al (2007) Effects of the adenosine A2A receptors antagonist SCH 58261 on cyclooxygenase-2 expression, glial activation and BDNF avaibility in a rat model of striatal neurodegeneration. J Neuropathol Exp Neurol 66:363–371
Lynch G, Kramar EA, Rex CS et al (2007) Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington’s disease. J Neurosci 27:4424–4434
Zuccato C, Liber D, Ramos C et al (2005) Progressive loss of BDNF in a mouse model of Huntington’s disease and rescue by BDNF delivery. Pharmacol Res 52:133–139
Pang TYC, Stam NC, Nithianantharajah J et al (2006) Differential effects of voluntary physical exercise on behavioural and brain-derived neurotrophic factor expression deficits in Huntington’s disease transgenic mice. Neuroscience 141:569–584
Cha JH, Frey AS, Alsdorf SA et al (1999) Altered neurotransmitter receptor expression in transgenic mouse models of Huntington’s disease, Philos. Trans. R. Soc. Lond. B Biol Sci 354 981–989
Tarditi A, Camurri A, Varani K et al (2006) Early and transient alteration of adenosine A(2A) receptor signaling in a mouse model of Huntington disease. Neurobiol Dis 23:44–53
Kovalchuk Y, Holthoff K, Konnerth A (2004) Neurotrophin action on a rapid timescale. Current Opinion in Neurobiol 14:558–563
Pousinha PA, Diogenes MJ, Ribeiro JA et al (2006) Triggering of BDNF facilitatory action on neuromuscular action transmission by adenosine A2A receptors. Neurosci Lett 404:143–147
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Potenza, R.L., Tebano, M.T., Martire, A. et al. Adenosine A2A receptors modulate BDNF both in normal conditions and in experimental models of Huntington’s disease. Purinergic Signalling 3, 333–338 (2007). https://doi.org/10.1007/s11302-007-9066-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-007-9066-y