Abstract
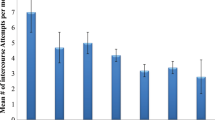
The chronological distribution of sexual intercourses in a group of patients treated with tadalafil versus placebo for 3 months was evaluated. In total, 120 patients with ED were randomized in two groups and treated, respectively, with one pill of tadalafil 20 mg or placebo on Tuesday and on Friday. After 3 months, we collected data using IIEF and SEP diaries. After 3 months, IIEF score and percentages of success SEP diaries increased in the tadalafil group (<0.01) versus placebo group. Considering all the successful intercourses of the 3 months of tadalafil assumption, the highest percentages were reported within 6–12 h range (35%) and 12–24 h range (28%). In tadalafil group, 41% of patients reported their first successful intercourse between 1 and 6 h and 78% of patients reported the recovery of spontaneous erections. In conclusion, after carrying out the first sexual attempt between 1 and 6 h, patients engaged in sexual activity between 6 and 24 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
McKinlay JB . The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res 2000; 12: S6.
Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP . Molecular mechanism of cGMP mediated smooth muscle relaxation. J Cell Physiol 2000; 184: 409.
Porst H . IC351 (tadalafil, Cialis). Update on clinical experience. Int J Impot Res 2002; 14(Suppl 1): S57–S64.
Padma-Nathan H, McMurray JG, Pullman WE . On demand IC351 (Cialis™) enhances erectile function in patients with erectile dysfunction. Int J Impot Res 2001; 13: 2–9.
Padma-Nathan H, Giuliano F . Oral drug therapy for erectile dysfunction. Urol Clin N Am 2001; 28: 321–334.
Angulo I . IC351 enhances NO-mediated relaxation of human arterial and trabecular penile smooth muscle. Eur Urol 2001; 39(Suppl 5): 106,, (abstract 415).
Giuliano F, Varanese L . Tadalafil: a novel treatment for erectile dysfunction. Eur Heart J 2002; 4(Suppl H): H24–H31.
Brock G, Iglesias J, Toulouse K . Efficacy and safety of IC351 treatment for ED. Eur Urol 2001; 39(Suppl 5): 106, (abstract).
Brock G et al. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol 2002; 168: 1332–1336.
Padma-Nathan H et al. Tadalafil (IC351) provides prompt and extended period of response for the treatment of men with ED. Int J Impot Res 2001; 13(Suppl 5): S64.
Rosen R et al. The International Index of Erectile Function (IIEF): a multidimensional scale fir assessment of erectile dysfunction. Urology 1997; 49: 822–830.
Cappelleri JC et al. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology 1999; 54: 346.
Hanson-Divers C et al. Health outcomes variables important to patients in the treatment of erectile dysfunction. J Urol 1998; 159: 1541.
Patterson B et al. Dose-normalized pharmacokinetics of single dose tadalafil (IC351) in healthy volunteers. Int J Impot Res 2001; 13(Suppl 5): S63.
Patterson B et al. The effect of intrinsic and extrinsic factors on the pharmacokinetic properties of tadalafil (IC351). Int J Impot Res 2001; 13(Suppl 5): S62.
Porst H et al. Tadalafil allows men with erectile dysfunction to have successful intercourse up to 36 h postdose. J Urol 2002; 167(Suppl): 177.
Jannini E, Lenzi A, Wagner G . New perspectives in the pharmacotherapy of erectile dysfunction. Drugs 2003; 6: 1165–1172.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Rose, A., Gallo, F. & Carmignani, G. Evaluation of sexual activity in patients treated with tadalafil: a randomized prospective placebo-controlled trial. Int J Impot Res 17, 76–79 (2005). https://doi.org/10.1038/sj.ijir.3901265
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijir.3901265
Keywords
This article is cited by
-
Cutaneous microcirculatory function predicts the responsiveness to tadalafil in patients with erectile dysfunction and coronary artery disease
International Journal of Impotence Research (2008)