Abstract
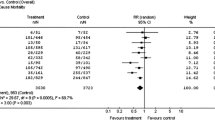
The Dual Chamber and VVI Implantable Defibrillator (DAVID) trial randomized 506 patients and tested the hypothesis that the dual-chamber pacing mode would produce improved hemodynamics and would in turn reduce congestive heart failure, heart failure hospitalizations, heart failure deaths, atrial fibrillation, strokes, ventricular arrhythmias, and total mortality compared to backup ventricular pacing in patients indicated for implantable defibrillator therapy. Patients had either primary prevention indications (47%) or secondary prevention indications (53%) for implantable defibrillator therapy but had no indications for bradycardia pacemaker support. All the patients had moderate to severe left ventricular dysfunction with a left ventricular ejection fraction of 40% or less (mean = 27%) and were consistently treated with angiotensin converting enzyme inhibitors or angiotensin II receptor blockers (86%) and beta adrenergic blocking agents (85%). The primary combined endpoint of hospitalization for congestive heart failure or death was paradoxically increased and statistically significant (p = 0.03) at one year in the patients paced in the dual chamber mode (22.6%) compared to patients randomized to ventricular backup pacing (13.3%). Both heart failure hospitalization and mortality contributed outcome.
Another perspective would consider this a randomized controlled study of presence or absence of pacemaker therapy in patients with left ventricular dysfunction and indications for implantable defibrillator therapy. Ventricular backup pacing produced less than 3% ventricular and no atrial pacing, while dual chamber pacing produced approximately 60% atrial and ventricular paced heart beats. The poor outcome in the dual chamber paced group correlated with the percentage of right ventricular pacing and suggests that right ventricular pacing caused ventricular dyssynchrony. The poor outcome associated with right ventricular pacing compared to intrinsic activation in the control group of the DAVID trial is reminiscent of the poor outcome associated with prolonged intraventricular conduction activation in the control groups compared to biventricular pacing in the intervention groups of the cardiac resynchronization trials.
The direct conclusion from these results are that patients with indications for implantable defibrillators and no indication for pacing should not be paced in the dual chamber pacing mode. It is not appropriate to conclude that only single chamber implantable defibrillators should be implanted. There are other potential advantages to having an implanted atrial lead including improved secondary outcomes. However the DAVID trial results suggest that the dual chamber paced mode was not associated with improved quality of life or decreased frequency of hospitalization, inappropriate shocks from the defibrillator or atrial fibrillation.
The more important question is what is the optimal pacing mode in these patients? The AAIR mode is under investigation in the DAVID II study in an attempt to identify a pacing mode that preserves atrio-ventricular synchrony, normal atrio-ventricular timing, prevents bradycardia and also prevents right ventricular stimulation.
Caution should be taken to not directly apply these results to patients with either an indication for pacemaker therapy or to patients with an indication for cardiac resynchronization therapy since patients from neither population were included. However, considering the large magnitude of the deleterious effects associated with dual chamber pacing in the DAVID trial future studies should explore the possibility that left ventricular stimulation may be the only pacing mode capable of preventing bradycardia without increasing death and congestive heart failure.
Similar content being viewed by others
References
GregoratosG, Abrams J, Epstein AE, Freedman RA, Hayes DL, Hlatky MA, Kerber RE, Naccarelli GV, Schoenfeld MH, Silka MJ, Winters SL, Gibbons RJ, Antman EM, Alpert JS, Hiratzka LF, Faxon DP, Jacobs AK, Fuster V, Smith SC, Jr. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines). Circulation 2002;106(16):2145–2161.
Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A. The DAVID Trial Investigators. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator. JAMA 2002;288(24):3115–3123.
Wilkoff BL. Should all patients receive dual chamber pacing ICDs? The rationale for the DAVID trial. Curr Control Trials Cardiovasc Med 2001;2:215–217.
AntiarrhythmicsVersus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med 1997;337:1576–1583.
Connolly SJ, Gent M, Roberts RS, Dorian P, RoyD, Sheldon RS, Mitchell LB, Green MS, Klein GJ, O'Brien B. Canadian implantable defibrillator study (CIDS): A randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation 2000;101(11):1297–1302.
Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest. The Cardiac Arrest Study Hamburg (CASH). Circulation 2000;102(7):748–754.
Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee R, Marinchak RA, Flaker G, Schron E, Orav EJ, Hellkamp AS, Greer S, McAnulty J, Ellenbogen K, Ehlert F, Freedman RA, Estes NA 3rd, Greenspon A, Goldman L. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 2002;346(24):1854–1862.
Lamas GA, Orav EJ, Stambler BS, Ellenbogen KA, Sgarbossa EB, Huang SK, Marinchak RA, Estes, NA 3rd, Mitchell GF, Lieberman EH, Mangione CM Goldman L, Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. Pacemaker Selection in the Elderly Investigators. N Engl J Med 1998;338(16):1097–1104.
Connolly SJ, Kerr CR, Gent M, Roberts RS, Yusuf S, Gillis AM, Sami MH, Talajic M, Tang AS, Klein GJ, Lau C, Newman DM. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med 2000;342(19):1385–1391.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wilkoff, B.L., the DAVID Trial Investigators The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial: Rationale, Design, Results, Clinical Implications and Lessons for Future Trials. Card Electrophysiol Rev 7, 468–472 (2003). https://doi.org/10.1023/B:CEPR.0000023165.20987.b1
Issue Date:
DOI: https://doi.org/10.1023/B:CEPR.0000023165.20987.b1