Key Points
-
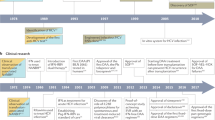
Hepatitis C virus (HCV) infection is an emerging global epidemic. The development of therapeutic and preventive strategies is a formidable challenge for biotechnology and pharmaceutical companies owing to the absence of adequate animal models and tissue-culture systems for viral propagation and reconstitution of the virus life cycle and pathogenesis. Despite these obstacles, remarkable progress has been made through collaborative efforts between basic researchers, clinicians and the pharmaceutical indutry.
-
Interferon-α (IFN-α)-based therapies remain the treatment of choice for hepatitis C. To improve the efficacy and safety of IFN therapies, some companies are focusing on modification of the IFN-α molecule, chemically or genetically, to develop long-lasting IFN molecules, whereas others are investigating combination therapies with known and new antivirals. However, most patients do not respond well to the existing IFN-based therapies, and alternative therapeutic interventions are desperately needed for these patients.
-
Several advances in the development of anti-HCV therapeutics have come about owing to: the definition of molecular clones that are infectious in the chimpanzee animal model of HCV infection; the availability of the three-dimensional structures of several of the key virally encoded enzymes; and the recent development of HCV-replicon systems in Huh-7 cells.
-
In vitro assays have been developed to examine viral enzymatic activities for the testing and development of antiviral agents, primarily targeting specific processes that are essential to HCV replication: virus translation; processing of the viral polyprotein protein; and viral RNA replication.
-
Inhibitors of the HCV internal ribosome-entry site (IRES), non-structural protein 3 (NS3) protease and NS5B polymerase are in clinical development at present. Several small-molecule compounds, which inhibit viral replication, and a few prophylactic vaccines, are under investigation for hepatitis C.
-
Cellular genes, pathways or processes required for HCV replication represent an attractive class of genes for small-molecule targeting to control viral replication. Therapeutic strategies that are being developed focus on depleting intracellular pools of guanine nucleotides by inhibiting the cellular IMPDH. Other potential targets include disruption of the host-cell glycosylation machinery for viral morphogenesis and secretion through inhibition of endoplasmic reticulum (ER) α-glucosidases and association of HCV NS proteins with the ER membranes and/or host-cell proteins.
-
Several therapies that preserve the cell structure, or prevent or reverse fibrosis and cirrhosis that are caused by chronic hepatitis C, or prevent the recurrence of hepatitis C after liver-transplant surgery are also under clinical development.
Abstract
Chronic infection with hepatitis C virus (HCV) is an emerging global epidemic. The development of effective HCV antiviral therapeutics continues to be a daunting challenge owing to the absence of adequate animal models and tissue-culture systems for analysis and propagation of the virus. Despite these obstacles, inhibitors of the replicative elements of HCV, immune modulators and non-specific hepatoprotective agents are being pursued and exciting progress has been made. Successful therapeutic intervention of HCV will probably require combination approaches and new approaches, including host drug discovery targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Choo, Q. L. et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244, 359–362 (1989). The original report to describe the molecular cloning of the HCV virus from a complementary DNA library made from blood containing the non-A, non-B hepatitis agent.
Hoofnagle, J. H. Hepatitis C — the clinical spectrum of disease. Hepatology 26, S15–S20 (1997).
Memon, M. I. & Memon, M. A. Hepatitis C: an epidemiological review. J. Viral Hepatitis 9, 84–100 (2002).
Anonymous. Hepatitis C — global prevalence (update). World Health Org. Weekly Epidemiol. Rec. 75, 18–19 (2000).
Willems, M., Metselaar, H. J., Tilanus, H. W., Schalm, S. W. & de Man, R. A. Liver transplantation and hepatitis C. Transplant Int. 15, 61–72 (2002).
Pollard, R. B. Analogy of human immunodeficiency virus to hepatitis C virus: the human immunodeficiency model. Am. J. Med. 107, 41S–44S (1999).
Rehermann, B. Interaction between the hepatitis C virus and the immune system. Semin. Liv. Dis. 20, 127–141 (2000).
Di Bisceglie, A. M., McHutchinson, J. & Rice, C. M. New therapeutic strategies for hepatitis C. Hepatology 35, 224–231 (2002). An excellent short review about the most recent developments in HCV therapeutic strategies.
Wang, Q. M. & Heinz, B. A. Recent advances in prevention and treatment of hepatitis C virus infections. Prog. Drug Res. 55, 1–32 (2000).
Dymock, B. W., Jones, P. S. & Wilson, F. X. Novel approaches to the treatment of hepatitis C virus infection. Antivir. Chem. Chemother. 11, 79–96 (2000).
Ideo, G. & Bellobuono, A. New therapies for the treatment of chronic hepatitis C. Curr. Pharm. Des. 8, 959–966 (2002).
Locarnini, S. A. & Bartholomeusz, A. Advances in hepatitis C: what is coming in the next 5 years? J. Gastroenterol. Hepatol. 17, 442–447 (2002).
Myles, D. C. Recent advances in the discovery of small molecule therapies for HCV. Curr. Opin. Drug Discov. Dev. 4, 411–416 (2001). A review about the advances in small-molecule antivirals for the treatment of HCV.
Bartenschlager, R. & Lohmann, V. Novel cell culture systems for the hepatitis C virus. Antiviral Res. 52, 1–17 (2001). A comprehensive review about the developments in HCV cell-culture systems, including the replicon cell-based system, written by the inventors of the HCV-replicon system.
Mercer, D. F. et al. Hepatitis C virus replication in mice with chimeric human livers. Nature Med. 7, 927–933 (2001). A description of an elegant and novel mouse model that supports prolonged HCV infection, which could be serially passaged through three generations of mice, confirming both synthesis and release of infectious viral particles. This is the first mouse model to be suitable for studying human HCV in vivo.
Ilan, E. et al. The hepatitis C virus (HCV)-Trimera mouse: a model for evaluation of agents against HCV. J. Infect. Dis. 185, 153–161 (2002).
Robertson, B. et al. Classification, nomenclature, and database development for hepatitis C virus (HCV) and related viruses: proposals for standardization. International Committee on Virus Taxonomy. Arch. Virol. 143, 2493–2503 (1998).
Rosenberg, S. Recent advances in the molecular biology of hepatitis C virus. J. Mol. Biol. 313, 451–464 (2001).
Friebe, P., Lohmann, V., Krieger, N. & Bartenschlager, R. Sequences in the 5′ nontranslated region of hepatitis C virus required for RNA replication. J. Virol. 75, 12047–12057 (2001). A description of sequences that are necessary for HCV RNA replication, analysing functions of the different 3′NTR domains.
Friebe, P. & Bartenschlager, R. Genetic analysis of sequences in the 3′ nontranslated region of hepatitis C virus that are important for RNA replication. J. Virol. 76, 5326–5338 (2002).
Reed, K. E. & Rice, C. M. Overview of hepatitis C virus genome structure, polyprotein processing, and protein properties. Hep. C Viruses 242, 55–84 (2000).
Carrere-Kremer, S. et al. Subcellular localization and topology of the p7 polypeptide of hepatitis C virus. J. Virol. 76, 3720–3730 (2002).
Walewski, J. L., Keller, T. R., Stump, D. D. & Branch, A. D. Evidence for a new hepatitis C virus antigen encoded in an overlapping reading frame. RNA-A Pub. RNA Soc. 7, 710–721 (2001).
Xu, Z. M. et al. Synthesis of a novel hepatitis C virus protein by ribosomal frameshift. EMBO J. 20, 3840–3848 (2001).
Shirota, Y. et al. Hepatitis C virus (HCV) NS5A binds RNA-dependent RNA polymerase (RdRP) NS5B and modulates RNA-dependent RNA polymerase activity. J. Biol. Chem. 277, 11149–11155 (2002).
Bartenschlager, R. & Lohmann, V. Replication of hepatitis C virus. J. Gen. Virol. 81, 1631–1648 (2000). A review about all aspects of the HCV life cycle and model systems.
Pileri, P. et al. Binding of hepatitis C virus to CD81. Science 282, 938–941 (1998).
Monazahian, M. et al. Low density lipoprotein receptor as a candidate receptor for hepatitis C virus. J. Med. Virol. 57, 223–229 (1999).
Agnello, V., Abel, G., Elfahal, M., Knight, G. B. & Zhang, Q. X. Hepatitis C virus and other Flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl Acad. Sci. USA 96, 12766–12771 (1999).
Neumann, A. U. et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-α therapy. Science 282, 103–107 (1998). A solid study of HCV dynamics in patients before and after IFN treatment.
Bukh, J., Miller, R. H. & Purcell, R. H. Genetic heterogeneity of hepatitis C virus — quasispecies and genotypes. Semin. Liv. Dis. 15, 41–63 (1995).
Zein, N. N. Clinical significance of hepatitis C virus genotypes. Clin. Microbiol. Rev. 13, 223–235 (2000).
Herion, D. & Hoofnagle, J. H. The interferon sensitivity determining region — all hepatitis C virus isolates are not the same. Hepatology 25, 769–771 (1997).
Tan, S. L. & Katze, M. G. How hepatitis C virus counteracts the interferon response: the jury is still out on NS5A. Virology 284, 1–12 (2001).
Farci, P. & Purcell, R. H. Clinical significance of hepatitis C virus genotypes and quasispecies. Semin. Liv. Dis. 20, 103–126 (2000). A review that describes the effects of HCV genotypes and quasispecies on treatment outcome in the clinic.
Dammacco, F. et al. The lymphoid system in hepatitis C virus infection: autoimmunity, mixed cryoglobulinemia, and overt B-cell malignancy. Semin. Liv. Dis. 20, 143–157 (2000).
Hoofnagle, J. H. et al. Treatment of chronic non-A, non-B hepatitis with recombinant human α-interferon. A preliminary report. N. Engl. J. Med. 315, 1575–1578 (1986).
Scott, L. J. & Perry, C. M. Interferon-α2b plus ribavirin — a review of its use in the management of chronic hepatitis C. Drugs 62, 507–556 (2002).
Lau, J. Y. N., Tam, R. C., Liang, T. J. & Hong, Z. Mechanism of action of ribavirin in the combination treatment of chronic HCV infection. Hepatology 35, 1002–1009 (2002).
Bekkering, F. C., Brouwer, J. T., Hansen, B. E. & Schalm, S. W. Hepatitis C viral kinetics in difficult to treat patients receiving high dose interferon and ribavirin. J. Hepatol. 34, 435–440 (2001).
Tsutsumi, M., Takada, A., Takase, S. & Sawada, M. Effects of combination therapy with interferon and ofloxacin on chronic type C hepatitis — a pilot study. J. Gastroenterol. Hepatol. 11, 1006–1011 (1996).
Komatsu, M. et al. Pilot study of ofloxacin and interferon-α combination therapy for chronic hepatitis C without sustained response to initial interferon administration. Can. J. Gastroenterol. 11, 507–511 (1997).
Moscarella, S. et al. Interferon and thymosin combination therapy in naive patients with chronic hepatitis C — preliminary results. Liver 18, 366–369 (1998).
Brillanti, S., Levantesi, F., Masi, L., Foli, M. & Bolondi, L. Triple antiviral therapy as a new option for patients with interferon nonresponsive chronic hepatitis C. Hepatology 32, 630–634 (2000).
Zilly, M. et al. Triple antiviral re-therapy for chronic hepatitis C with interferon-α, ribavirin and amantadine in nonresponders to interferon-α and ribavirin. Eur. J. Med. Res. 7, 149–154 (2002).
Kozlowski, A., Charles, S. A. & Harris, J. M. Development of pegylated interferons for the treatment of chronic hepatitis C. Biodrugs 15 419–429 (2001).
Zeuzem, S. et al. PEGinterferon-α2a in patients with chronic hepatitis C. N. Engl. J. Med. 343, 1666–1672 (2000).
Heathcote, E. J. et al. PEG-interferon-α2a in patients with chronic hepatitis C and cirrhosis. N. Engl. J. Med. 343, 1673–1680 (2000).
Manns, M. P. et al. PEG-interferon-α2b plus ribavirin compared with interferon-α2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358, 958–965 (2001). References 47–49 describe results of a clinical trial that was done with PEGylated IFN alone or in combination with ribavirin, compared with unmodified IFN.
Enomoto, M. et al. Dynamics of hepatitis C virus monitored by real-time quantitative polymerase chain reaction during first 2 weeks of IFN-β treatment are predictive of long-term therapeutic response. J. Interferon Cytokine Res. 22, 389–395 (2002).
Barbaro, G. et al. Intravenous recombinant interferon-β versus interferon-α2b and ribavirin in combination for short-term treatment of chronic hepatitis C patients not responding to interferon-α. Scand. J. Gastroenterol. 34, 928–933 (1999).
Kumashiro, R. et al. Interferon-γ brings additive anti-viral environment when combined with interferon-α in patients with chronic hepatitis C. Hepatol. Res. 22, 20–26 (2002).
Barbaro, G. & Barbarini, G. Consensus interferon for chronic hepatitis C patients with genotype 1 who failed to respond to, or relapsed after, interferon-α2b and ribavirin in combination: an Italian pilot study. Eur. J. Gastroenterol. Hepatol. 14, 477–483 (2002).
Chang, C. C. et al. Evolution of a cytokine using DNA family shuffling. Nature Biotechnol. 17, 793–797 (1999).
Maier, I. & Wu, G. Y. Hepatitis C and HIV co-infection: a review. World J. Gastroenterol. 8, 577–579 (2002).
Falck-Ytter, Y. et al. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann. Intern. Med. 136, 288–292 (2002).
De Francesco, R. et al. Biochemical and immunologic properties of the nonstructural proteins of the hepatitis C virus: implications for development of antiviral agents and vaccines. Sem. Liv. Dis. 20, 69–83 (2000). A review that describes the function and properties of HCV NS proteins and their implications for drug and vaccine discovery.
Darke, P. L., Jacobs, A. R., Waxman, L. & Kuo, L. C. Inhibition of hepatitis C virus NS2/3 processing by NS4A peptides — implications for control of viral processing. J. Biol. Chem. 274, 34511–34514 (1999).
Thibeault, D., Maurice, R., Pilote, L., Lamarre, D. & Pause, A. In vitro characterization of a purified NS2/3 protease variant of hepatitis C virus. J. Biol. Chem. 276, 46678–46684 (2001).
Grakoui, A., McCourt, D. W., Wychowski, C., Feinstone, S. M. & Rice, C. M. A second hepatitis C virus-encoded proteinase. Proc. Natl Acad. Sci. USA 90, 10583–10587 (1993).
Hijikata, M. et al. Two distinct proteinase activities required for the processing of a putative nonstructural precursor protein of hepatitis C virus. J. Virol. 67, 4665–4675 (1993).
Whitney, M. et al. A collaborative screening program for the discovery of inhibitors of HCV NS2/3 cis-cleaving protease activity. J. Biomol. Screening 7, 149–154 (2002). References 58–62 describe the identification, in vivo validation and in vitro assay development for the NS2–NS3 protease activity of HCV. It is now possible to screen this novel protease target for small-molecule inhibitors.
Kolykhalov, A. A., Mihalik, K., Feinstone, S. M. & Rice, C. M. Hepatitis C virus-encoded enzymatic activities and conserved RNA elements in the 3′ nontranslated region are essential for virus replication in vivo. J. Virol. 74, 2046–2051 (2000).
Yao, N. H., Reichert, P., Taremi, S. S., Prosise, W. W. & Weber, P. C. Molecular views of viral polyprotein processing revealed by the crystal structure of the hepatitis C virus bifunctional protease-helicase. Structure 7, 1353–1363 (1999).
Ingallinella, P. et al. Prime site binding inhibitors of a serine protease: NS3/4A of hepatitis C virus. Biochemistry 41, 5483–5492 (2002).
Zhang, R. M., Durkin, J. P. & Windsor, W. T. Azapeptides as inhibitors of the hepatitis C virus NS3 serine protease. Bioorg. Med. Chem. Lett. 12, 1005–1008 (2002).
Slater, M. J. Design and synthesis of pyrrolidine-5,5-trans-lactams as novel inhibitors of hepatitis C virus protease. Antiviral Research — 15th International Conference, Prague, Czech Rebublic (2002).
Fukuda, K. et al. Isolation and characterization of RNA aptamers specific for the hepatitis C virus nonstructural protein 3 protease. Eur. J. Biochem. 267, 3685–3694 (2000).
Kumar, P. K. R. et al. Isolation of RNA aptamers specific to the NS3 protein of hepatitis C virus from a pool of completely random RNA. Virology 237, 270–282 (1997).
Yao, N. H. et al. Structure of the hepatitis C virus RNA helicase domain. Nature Struct. Biol. 4, 463–467 (1997).
Kang, L. W. et al. Crystallization and preliminary X-ray crystallographic analysis of the helicase domain of hepatitis C virus NS3 protein. Acta Crystallograph. D 54, 121–123 (1998).
Levin, M. K. & Patel, S. S. The helicase from hepatitis C virus is active as an oligomer. J. Biol. Chem. 274, 31839–31846 (1999).
Pang, P. S., Jankowsky, E., Planet, P. J. & Pyle, A. M. The hepatitis C viral NS3 protein is a processive DNA helicase with cofactor enhanced RNA unwinding. EMBO J. 21, 1168–1176 (2002).
Tanji, Y., Hijikata, M., Satoh, S., Kaneko, T. & Shimotohno, K. Hepatitis C virus-encoded nonstructural protein NS4A has versatile functions in viral protein processing. J. Virol. 69, 1575–1581 (1995).
Yang, S. H., Lee, C. G., Song, M. K. & Sung, Y. C. Internal cleavage of hepatitis C virus NS3 protein is dependent on the activity of NS34A protease. Virology 268, 132–140 (2000).
Kolykhalov, A. A. et al. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science 277, 570–574 (1997). A description of an infectious HCV clone that can transmit HCV by injection of its RNA into the liver of chimpanzees. This clone was infectious only after a consensus clone was derived from many patient isolates, implying that not every virus in a patient is infectious. This work defines the structure of a functional HCV-genome RNA, and proves that HCV alone is sufficient to cause disease.
Leveque, V. J. -P. & Wang, Q. M. RNA-dependent RNA polymerase encoded by hepatitis C virus: biomedical applications. Cell. Mol. Life Sci. 59, 909–919 (2002).
Bressanelli, S. et al. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Proc. Natl Acad. Sci. USA 96, 13034–13039 (1999).
Lesburg, C. A. et al. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nature Struct. Biol. 6, 937–943 (1999).
Qin, W. P. et al. Oligomeric interaction of hepatitis C virus NS5B is critical for catalytic activity of RNA-dependent RNA polymerase. J. Biol. Chem. 277, 2132–2137 (2002).
Cheney, I. W. et al. Mutations in NS5B polymerase of hepatitis C virus: impacts on in vitro enzymatic activity and viral RNA replication in the subgenomic replicon cell culture. Virology 297, 298–306 (2002).
De Francesco, R. Diketobutanoic acids as HCV Pol inhibitors. 7th International Meeting on Hepatitis C Virus and Related Viruses (Molecular Virology and Pathogenesis), Gold Coast, Queensland, Australia (2000).
Moradpour, D. et al. Functional properties of a monoclonal antibody inhibiting the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 277, 593–601 (2002). A description of a monoclonal antibody that potently and specifically inhibits HCV RdRP in vitro . This could be a new avenue towards developing an HCV therapeutic.
Biroccio, A., Hamm, J., Incitti, I., De Francesco, R. & Tomei, L. Selection of RNA aptamers that are specific and high-affinity ligands of the hepatitis C virus RNA-dependent RNA polymerase. J. Virol. 76, 3688–3696 (2002).
Usman, N. & Blatt, L. R. M. Nuclease-resistant synthetic ribozymes: developing a new class of therapeutics. J. Clin. Invest. 106, 1197–1202 (2000).
Macejak, D. G. et al. Inhibition of hepatitis C virus (HCV)-RNA-dependent translation and replication of a chimeric HCV poliovirus using synthetic stabilized ribozymes. Hepatology 31, 769–776 (2000).
Zhang, H. et al. Antisense oligonucleotide inhibition of hepatitis C virus (HCV) gene expression in livers of mice infected with an HCV vaccinia virus recombinant. Antimicrob. Agents Chemother. 43, 347–353 (1999).
Brown-Driver, V., Eto, T., Lesnik, E., Anderson, K. P. & Hanecak, R. C. Inhibition of translation of hepatitis C virus RNA by 2′-modified antisense oligonucleotides. Antisense Nucleic Acid Drug Dev. 9, 145–154 (1999).
Witherell, G. ISIS-14803 (Isis Pharmaceuticals). Curr. Opin. Invest. Drugs 2, 1523–1529 (2001).
Lambot, M. et al. Reconstitution of hepatitis C virus envelope glycoproteins into liposomes as a surrogate model to study virus attachment. J. Biol. Chem. 277, 20625–20630 (2002).
Wellnitz, S. et al. Binding of hepatitis C virus-like particles derived from infectious clone H77C to defined human cell lines. J. Virol. 76, 1181–1193 (2002).
Tseng, C. T. K. & Klimpel, G. R. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J. Exp. Med. 195, 43–49 (2002).
Crotta, S. et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J. Exp. Med. 195, 35–41 (2002).
Weiner, A. J. et al. Intrahepatic genetic inoculation of hepatitis C virus RNA confers cross-protective immunity. J. Virol. 75, 7142–7148 (2001).
Major, M. E. et al. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J. Virol. 76, 6586–6595 (2002).
Bassett, S. E. et al. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology 33, 1479–1487 (2001).
Bukh, J., Forns, X., Emerson, S. U. & Purcell, R. H. Studies of hepatitis C virus in chimpanzees and their importance for vaccine development. Intervirology 44, 132–142 (2001).
Lechmann, M. & Liang, T. J. Vaccine development for hepatitis C. Semin. Liv. Dis. 20, 211–226 (2000).
Markland, W., McQuaid, T. J., Jain, J. & Kwong, A. D. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with α-interferon. Antimicrob. Agents Chemother. 44, 859–866 (2000).
Dwek, R. A., Butters, T. D., Platt, F. M. & Zitzmann, N. Targeting glycosylation as a therapeutic approach. Nature Rev. Drug Discov. 1, 65–75 (2002).
Block, T. M. et al. Treatment of chronic hepadnavirus infection in a woodchuck animal model with an inhibitor of protein folding and trafficking. Nature Med. 4, 610–614 (1998).
Mehta, A. et al. Inhibition of hepatitis B virus DNA replication by imino sugars without the inhibition of the DNA polymerase: therapeutic implications. Hepatology 33, 1488–1495 (2001).
Zitzmann, N. et al. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents. Proc. Natl Acad. Sci. USA 96, 11878–11882 (1999).
Durantel, D. et al. Study of the mechanism of antiviral action of iminosugar derivatives against bovine viral diarrhea virus. J. Virol. 75, 8987–8998 (2001).
Jordan, R. et al. Inhibition of host ER glucosidase activity prevents Golgi processing of virion-associated bovine viral diarrhea virus E2 glycoproteins and reduces infectivity of secreted Virions. Virology 295, 10–19 (2002).
Wu, S. F. et al. Antiviral effects of an iminosugar derivative on flavivirus infections. J. Virol. 76, 3596–3604 (2002).
Block, T. M. & Jordan, R. Iminosugars as possible broad spectrum anti hepatitis virus agents: the glucovirs and alkovirs. Antiviral Chem. Chemother. 12, 317–325 (2001).
Mottola, G. et al. Hepatitis C virus nonstructural proteins are localized in a modified endoplasmic reticulum of cells expressing viral subgenomic replicons. Virology 293, 31–43 (2002).
Schmidt-Mende, J. et al. Determinants for membrane association of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 276, 44052–44063 (2001).
Brass, V. et al. An amino-terminal amphipathic α-helix mediates membrane association of the hepatitis C virus nonstructural protein 5A. J. Biol. Chem. 277, 8130–8139 (2002).
Wolk, B. et al. Subcellular localization, stability, and trans-cleavage competence of the hepatitis C virus NS3–NS4A complex expressed in tetracycline-regulated cell lines. J. Virol. 74, 2293–2304 (2000).
Harada, T., Tautz, N. & Thiel, H. J. E2–p7 region of the bovine viral diarrhea virus polyprotein: processing and functional studies. J. Virol. 74, 9498–9506 (2000).
Griffin, S. Hexamerization of the hepatitis C virus p7 protein: possible fuction as a virus-encoded cation channel. 9th International Meeting on Hepatitis and Related Viruses, San Diego, USA (2002).
Hannon, G. J. RNA interference. Nature 418, 244–251 (2002).
Zamore, P. D. Ancient pathways programmed by small RNAs. Science 296, 1265–1269 (2002).
Novina, C. D. et al. siRNA-directed inhibition of HIV-1 infection. Nature Med. 8, 681–686 (2002).
Lee, N. S. et al. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nature Biotechnol. 20, 500–505 (2002).
Jacque, J. M., Triques, K. & Stevenson, M. Modulation of HIV-1 replication by RNA interference. Nature 418, 435–438 (2002).
Gitlin, L., Karelsky, S. & Andino, R. Short interfering RNA confers intracellular antiviral immunity in human cells. Nature 418, 430–434 (2002).
McCaffrey, A. P. et al. Gene expression — RNA interference in adult mice. Nature 418, 38–39 (2002).
Nelson, D. R., Lauwers, G. Y., Lau, J. Y. N. & Davis, G. L. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology 118, 655–660 (2000).
Muratori, L. & Gibellini, D. A new route to apoptosis in hepatitis C virus infection. J. Hepatol. 35, 814–815 (2001).
Tsantrizos, Y. S. et al. Macrocyclic peptides active against the hepatitis C virus. Int. Patent Appl. WO 00/59929 (2000).
Kim, J. L. et al. Hepatitis C virus NS3 RNA helicase domain with a bound oligonucleotide: the crystal structure provides insights into the mode of unwinding. Structure 6, 89–100 (1998).
Hashimoto, H. et al. Preparation of heterocyclic compounds as remedies for hepatitis C. Int. Patent Appl. WO 01/47883 (2001).
Acknowledgements
We would like to thank J. Colacino, M. López Lastra and our anonymous reviewers for reading this manuscript and providing helpful suggestions, and K. McKnight, M. Bures, J. Puglisi, Y. S. Tsantrizos, J. Tang, P. l. Caron, M. Wang and K.-L. Yu for providing the various Figures. Unfortunately, owing to space constraints, many citations and explanations have been limited.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Cancer.gov
LocusLink
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- FIBROSIS
-
A process that replaces lost parenchymal tissue, resulting in scar formation.
- CIRRHOSIS
-
A liver disease in which parenchymal tissues die and the liver becomes filled with fibrous tissue.
- HEPATOCELLULAR CARCINOMA
-
A malignant tumour of the liver that is seen in some people with long-term liver damage due to chronic hepatitis C or hepatitis B.
- VIRAL LOAD
-
The amount of virus that is present in the blood.
- CYTOTOXIC T LYMPHOCYTES
-
A subset of T lymphocytes that can kill body cells that have been infected by viruses or transformed by cancer.
- TH1 CELLS
-
T lymphocytes that produce cytokines to help inflammation and antiviral responses.
- TH2 CELLS
-
T lymphocytes that produce cytokines to help antibody responses.
- INTERFERONS
-
(IFN). Secreted cytokines that are known for their antiviral, antiproliferative and immunomodulatory activities. There are two types of IFN: type I (IFN-α, IFN-β, IFN-ω and IFN-τ) and type II (IFN-γ). There are at least 14 IFN-α genes, but only one IFN-β and IFN-γ gene has been reported so far.
- HCV REPLICON
-
A biscistronic DNA construct that contains a selectable marker gene and genes encoding HCV non-structural proteins, in which an HCV IRES and an EMCV IRES direct the translation of the marker gene and viral genes, respectively. Transfection of RNA transcribed from such constructs into the hepatoma cell line Huh-7 results in selectable, autonomously replicating HCV RNAs.
- ENVELOPE
-
A lipoprotein-bilayer outer membrane of many viruses. Often heavily glycosylated, envelope proteins usually function to identify and attach the virus to the cell-surface receptor, so that viral entry can occur.
- NUCLEOCAPSID
-
The coat (capsid) of a virus plus the enclosed nucleic-acid genome.
- VIRION
-
A mature infectious virus particle that exists freely outside the host cell.
- NUCLEOSIDE TRIPHOSPHATASE
-
An enzyme that hydrolyses nucleoside triphosphate (NTP) — a nucleotide that is of fundamental importance as a carrier of chemical energy in all living organisms — to drive energetically unfavourable biological processes.
- RNA HELICASE
-
An ATP-dependent enzyme that catalyses the unwinding of RNA helices.
- ENDOCYTOSIS
-
A process by which proteins or viral particles at the cell surface are internalized, being transported into the cell within membranous vesicles.
- NEGATIVE-STRAND RNA
-
Genomic viral RNA that is complementary to the messenger RNA (positive strand) that is produced during replication.
- PKR
-
A serine/threonine protein kinase and among the best-studied effectors of the host interferon (IFN)-induced antiviral and antiproliferative response system. In response to stress signals, including virus infection, the normally latent PKR becomes activated through autophosphorylation and dimerization and phosphorylates the eIF2α translation-initiation-factor subunit, leading to an inhibition of the intiation of mRNA translation.
- QUASISPECIES
-
A family of closely related, but slightly different, viral genomes. Viral genetic variants, derived from the original infecting virus, which are present during an infection.
- MIXED CRYOGLOBULINAEMIA
-
The presence of abnormal proteins called cryoglobulins in blood. Cryoglobulinaemia can cause damage to the kidneys.
- GLOMERULONEPHRITIS
-
A kidney disease that affects the capillaries of the glomeruli (the compact cluster of capillaries in the kidney that filter blood) — characterized by oedema, raised blood pressure and excess protein in the urine.
- LEUKOPAENIA
-
A decrease in the number of leukocytes (white blood cells).
- THROMBOCYTOPAENIA
-
A condition in which there is an abnormally small number of platelets in the circulating blood.
- SUSTAINED VIROLOGICAL RESPONSE
-
(SVR). The continued lack of detectable serum HCV RNA six months after the completion of treatment.
- LETHAL MUTAGENESIS
-
A process by which animal RNA viruses, given their high mutation frequencies, undergo a sharp decline in viability after a modest increase in mutation frequency that results from the promiscuous incorporation of nucleoside analogues, such as ribavirin triphosphate, by the viral RNA polymerase.
- HAEMOLYTIC ANAEMIA
-
A decrease in the normal level of erythocytes (red blood cells) in the bloodstream owing to the destruction (rather than underproduction) of red blood cells.
- PEGYLATION
-
A technique of conjugating polyethylene glycol (PEG) groups to proteins to increase their resistance to proteolytic degradation, improve their water solubility and reduce their antigenicity.
- ALBUMIN
-
A protein that is made in the liver, and the most abundant protein in the blood. A low albumin level is associated with liver cirrhosis.
- MOLECULAR BREEDING
-
In vitro recombination-based directed evolution to yield a high percentage of functional variants, as identified and evaluated using marker-assisted selection.
- PEPTIDOMIMETIC
-
A compound containing non-peptidic structural elements that can mimic or antagonize the biological action(s) of a natural parent peptide.
- STRUCTURE–ACTIVITY RELATIONSHIP
-
(SAR). The relationship between chemical structure and pharmacological activity for a series of compounds. Compounds are often classed together because they have structural characteristics in common, including shape, size, stereochemical arrangement and distribution of functional groups. Other factors contributing to the structure–activity relationship include chemical reactivity, electronic effects, resonance and inductive effects.
- RNA APTAMER
-
A single-stranded RNA oligonucleotide that assumes a specific, sequence-dependent shape and binds to a target protein on the basis of a lock-and-key fit between the two molecules.
- INNATE IMMUNE RESPONSE
-
A crucial response during the early phase of host defence against infection by pathogens, before the antigen-specific, adaptive immune response is induced.
- PRODRUG
-
A pharmacologically inactive compound that is converted to the active form of the drug by endogenous enzymes or metabolism. It is generally designed to overcome problems that are associated with stability, toxicity, lack of specificity or limited (oral) bioavailability.
Rights and permissions
About this article
Cite this article
Tan, SL., Pause, A., Shi, Y. et al. Hepatitis C therapeutics: current status and emerging strategies. Nat Rev Drug Discov 1, 867–881 (2002). https://doi.org/10.1038/nrd937
Issue Date:
DOI: https://doi.org/10.1038/nrd937
This article is cited by
-
A conserved RNA structural motif for organizing topology within picornaviral internal ribosome entry sites
Nature Communications (2019)
-
Chemical genetics-based development of small molecules targeting hepatitis C virus
Archives of Pharmacal Research (2017)
-
Identification of novel inhibitors of HCV NS3 protease genotype 3 subtype B through molecular docking studies of phytochemicals from Boerhavia diffusa L
Medicinal Chemistry Research (2017)
-
Structure–activity relationship studies on quinoxalin-2(1H)-one derivatives containing thiazol-2-amine against hepatitis C virus leading to the discovery of BH6870
Molecular Diversity (2015)
-
k nearest neighbor-molecular field analysis on human HCV NS5B polymerase inhibitors: 2,5-disubstituted imidazo[4,5-c]pyridines
Medicinal Chemistry Research (2013)