Summary
Halofantrine is a phenanthrenemethanol antimalarial that is effective against asexual forms of multidrug-resistant Plasmodium falciparum malaria. It has no action on gametocytes or hypnozoites in the liver. The drug is administered as a racemic mixture but the (+)- and (−)-enantiomers show no difference in activity in vitro. Three formulations for oral administration are available for human use, i.e. tablets, capsules and suspension. Toxicity studies in animals suggest that halofantrine has very low toxicity both in short term and long term animal studies, and there has been no evidence of mutagenicity in these studies.
Phase I, II and III clinical trials of halofantrine conducted in several tropical countries found the drug to be well tolerated and effective against multidrug-resistant P. falciparum malaria when 500mg was administered every 6 hours for 3 doses. The majority of clinical adverse effects reported, including nausea, vomiting, abdominal pain, diarrhoea, orthostatic hypotension, prolongation of QTc interval, pruritus and rash, have been mild and transient.
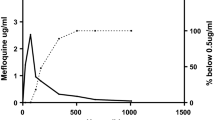
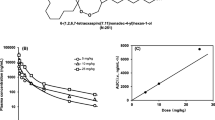
There is wide interindividual variation in halofantrine absorption. The maximal plasma concentration (Cmax) is achieved approximately 6 hours after oral administration. Bioavailability is not dose-proportional for doses over 500mg, but there is a dose-proportional increase in Cmax and area under the plasma concentration-time curve (AUC) for doses between 250 and 500mg. In patients with malaria the bioavailability of halofantrine is decreased. The mean half-life of absorption is 4 hours and Cmax is significantly lower than that obtained in healthy individuals. Furthermore, halofantrine absorption is enhanced when the drug is taken with fatty food. Therefore, halofantrine should be taken with food to ensure optimal absorption in patients with malaria. The terminal elimination half-life is 5 days in patients with malaria.
Halofantrine is biotransformed in the liver to its major metabolite N-debutylhalofantrine. Plasma concentrations of this metabolite are observed soon after administration of halofantrine, but in much lower concentrations. The elimination half-life is similar to that of halofantrine.
There have been increasing reports of halofantrine treatment failure, particularly in the eastern part of Thailand. The majority of treatment failures have been associated with incomplete drug absorption. The dose-dependent cardiotoxic effects (e.g. cardiac arrhythmia) are a major concern, particularly when the bioavailability of the drug cannot be predicted. Ongoing and future studies should aim at developing more appropriate drug formulation(s) and/or optimising dosage regimens. This will allow therapeutic concentrations to be achieved with minimum adverse effects, particularly cardiotoxicity.
Similar content being viewed by others
References
Rinehart J, Arnold J, Canfield CJ. Evaluation of two phenantherene methanols for antimalarial activity in man: WR 122,455 and WR 171,669. Am J Trop Med Hyg 1976; 25: 769–74
Childs GE, Lambros C, Notsch JD, et al. Comparison of in vitro and in vivo antimalarial activities of 9-phenanthrenecarbinols. Ann Trop Med Parasitol 1984; 78: 13–20
Desjardins RE, Canfield CJ, Haynes JD, et al. Quantitative assessment of antimalarial activity in vitro by a semi-automated microdilution teachnique. Antimicrob Agents Chemother 1979; 16: 710–8
Schmidt LH, Crosby R, Rasco J, et al. Antimalarial activities of various 9- phenanthrenemethanols with special attention to WR 122,455 and WR 171,669. Antimicrob Agents Chemother 1972; 14: 292–314
Osdene TS, Russell PB, Rane L. 2,4,7-triamino-6-ortho-substituted arylpteridines. A new series of potent antimalarial agents. J Med Chem 1967; 10: 431–6
Schmidt LH, Crosby R, Rasco J, et al. Antimalarial activities of various 9-phenan-threnemethanols with special attention to WR-122,455 and WR-171,669. Antimicrob Agents Chemother 1978; 14: 292–314
Boudreau EF, Pang LW, Dixon KE, et al. Treatment efficacy of halofantrine (WR 171,669) in initial field trials in Thailand. Bull World Health Organ 1988; 66: 227–35
Bunnag D, Viravan C, Looareesuwan S, Karbwang J, et al. Comparison of the efficacy of halofantrine and mefloquine in multidrug resistant P.falciparum malaria in Thailand. Abstract 84-C12. Bull Soc Fran Parasitol 1990; 8 Suppl. 1: 420
Horton RJ, Parr SN, Boker LC. Clinical experience with halofantrine in the treatment of malaria. Drugs Exp Clin Res 1990; 16: 497–503
Basco LK, Le Bras J. In vitro activity of halofantrine and its relationship to other standard antimalarial drugs against African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg 1992; 47(4): 521–7
Cosgriff TM, Pamplin CL, Canfield CJ, et al. Mefloquine failure in a case of falciparum malaria induced with a multi-drug resistant isolate in non-immune subject. Am J Trop Med Hyg 1985; 34: 692–3
Childs GE, Boudreau EF, Wimonwattrawatee T, et al. In vitro and clinical correlates of mefloquine resistance of Plasmodium falciparum in eastern Thailand. Am J Trop Med Hyg 1991; 44: 553–9
Felix R, Gay F, Lyagoubi A, et al. Cross resistance with mefloquine and halofantrine in a case of falciparum malaria contracted in Sierra Leone. Bull Soc Pathol Exot 1990; 83: 43–5
Le Bras J, Hatin I, Trape JF, et al. Susceptibility of Plasmodium falciparum to mefloquine in an urban area in Senegal. [abstract S4.D17]. Bull Soc Fran Parasitol 1990; 8 Suppl. 1: 461
Peters W, Robinson BL, Ellis DS. The chemotherapy of rodent malaria XLII. Halofantrine and halofantrine resistance. Ann Trop Med Parasitol 1987; 81: 639–46
Rojas Rivera L, Gay F, Bustos DG, et al. In vitro cross-resistance between mefloquine and halofantrine in Plasmodium falciparum induced by mefloquine [abstract S9.D31]. Bull Soc Fran Parasitol 1990; 8 Suppl. 1: 1118
Shanks GD, Watt G, Edstein MD, et al. Halofantrine for the treatment of mefloquine chemoprophylaxis failures in Plasmodium falciparum infections. Am J Trop Med Hyg 1991; 45: 488–91
Webster HK, Boudreau EF, Pavanand K, et al. Antimalarial drug susceptibility testing of Plasmodium falciparum in Thailand using a microdilution radioisotope method. Am J Trop Med Hyg 1985; 34: 228–35
Webster HK, Thaithong S, Pavanand K, et al. Cloning and characterization of mefloquine-resistant Plasmodium falciparum from Thailand. Am J Trop Med Hyg 1985; 34: 1022–7
Chutmongkonkul M, Maier WA, Seitz HM. Plasmodium falciparum: effect of chloroquine, halofantrine and pyrimethamine on the infectivity of gametocyte for Anopheles stephensi mosquitoes. Ann Trop Med Parasitol 1992; 86(2): 103–10
Fitch CD. Mode of action of antimalarial drugs. In: Malaria and the red cell. London: Pitman, 1983: 222–32
Warhurst DC. Antimalarial interaction with ferriporphyrin IX monomers and its relationship to activity of the blood schizontocides. Am J Trop Med Parasitol 1987; 81: 65–7
Hines JW, Sparacino CM, Cook CE. Interim Report August 1982, USAMRDC Contract no. DAMD-17-76-C-6075, 1982
Yanko WH. Synthesis Report. US Army Medical Research and Development Command Contract no. DADA-17-C-0098, 1972
Voyksner RD, Bursey JT, Hines JW, et al. A comparison of thermospray and direct liquid introduction high performance liquid chromatography/mass spectrometry for the analysis of candidate antimalarials. Biomed Mass-spectroscopy 1984; 11: 616–21
Keeratithakul D, Teja-Isavadharm P, Shanks GD, et al. An improved high-performance liquid chromatographic method for the simultaneous measurement of halofantrine and desbutylhalofantrine in human serum. Ther Drug Monit 1991; 13: 64–8
Milton KA, Ward SA, Edwards G. Determination of halofantrine and its principal metabolite desbutylhalofantrine in biological fluids by high performance liquid chromatography 433: J Chromatogr 1988; 433: 339–44
Camilleri P, Thorpe CJ. Fluorescence detection of the enantiomers of halofantrine at picomole levels using chiral high-performance liquid chromatography. J Chromatogr 1990; 519: 387–90
Gawienowski M, Benet L. Ion-paired liquid chromatographic method for the analysis of blood and plasma for the antimalarial drug halofantrine and its putative mono-debutylated metabolite. J Chromatogr 1988; 430: 412–9
Hines JW, Elkins PD, Cook CE, et al. Paired ion chromatography method for the analysis of a phenanthrenemethanol antimalarial in whole blood. J Pharm Sci 1985; 74: 433–7
Lin ET, Benet LZ, Upton RA, et al. WRAIR Report: Ion-paired liquid chromato-graphic method for the analysis of halofantrine (WR 171,669) and its putative metabolite, WR 178,460 in blood and plasma, 1985
Mberu WM, Muhia DK, Watkins DK. Measurement of halofantrine and its major metabolite desbutyl- halofantrine in plasma and blood by high-performance liquid chromatography: a new methodology. J Chromatogr 1992; 581: 156–60
Carroll FI, Berrang B, Linn CP. Resolution of antimalarial agents via complex formation with α- (2,4,5,7-tetranitro-9-fluorenyli-deneaminooxy) propionic acid. J Med Chem 1978; 21: 326–30
Gimenez F, Aubry AF, Farinotti R, et al. The determination of the enantiomers of halofantrine and monodesbutylhalofantrine in plasma and whole blood using sequential achiral/chiral high performance liquid chromatography. J Biomed Pharm Analysis 1992; 10: 245–50
Terefe H, Blaschke G. Direct determination of the enantiomers of halofantrine and its pharmacologically active metabolite N-desbutylhalofnatrine by high performance liquid chromatography. J Chromatogr 1993; 615: 347–51
Lee CC, Kinter LD, Sanyer JL, et al. Interim Report no. 65, 3 October 1972. US Army Medical Research and Development Command Contract no. DAMD-17-83-C-3004
Lee CC, Kintner LD, Sanyer JL, et al. Interim Report no. 66. Midwest Research Institute, Kansas City, Missouri 1972, US Army Medical Research and Development Command Contract no. DA-49-193-MD-2759
Hodgson JR, Kowalski MA, Goethem DV, et al. Interim Report no. 124. 31 August 1977. US Army Medical Research and Development Contract no. DAMD-17-74-C-4036, 1977
San Sebastian JR. SK&F Report no. TPOO5BF. August 1986. IND no. 9847; Suppl. 12, 1986
Sorg RM. SK&F Report no. TP006BF; August 1986. IND no. 9847. Suppl. 12, 1986
Loizeaux PS, Grenan MM. Research Report; WAIR, 7 Dec 1977. IND no. 9847, Suppl. 5
Gaffar AG. Final report. 29 February 1980, US Army Medical Research and Development Command Contract no. DAMD-17-79-C-9025
Horton RJ, Parr SN. Halofantrine: an overview of efficacy and safety. Parasitology Today 1989; (Suppl.): 65–79
Parkinson D, Balmer V, Ajdukiewicz A, et al. The effectiveness of halofantrine for the treatment of acute malaria in adults in the Solomon Islands. Parasitology Today 1989; (Suppl.): 27–35
Baudon D, Bernard J, Moulia-Pelat JP, et al. Halofantrine to prevent falciparum malaria on return from malarious areas. Lancet 1990; 336: 337
Bunnag D, Viravan C, Karbwang J, et al. Clinical trials with halofantrine in acute uncomplicated falciparum malaria in Thailand. Southeast Asian J Trop Med Public Health 1993; 24: 43–8
Ezeamuzie IC, Igbigbi PS, Ambakederemo AW, et al. Halofantrine-induced pruritus amongst subjects who itch to chloroquine. J Trop Med Hyg 1991; 94: 184–8
Vachon F, Fajac I, Gachot B. Halofantrine and acute intravascular haemolysis. Lancet 1992; 340: 909–10
ter Kuile FO, Dolan G, Nosten F, et al. Halofantrine versus mefloquine in treatment of multidrug resistant falciparum malaria. Lancet 1993; 341: 1044–9
Nosten F, ter Kuile FO, Luxemburger C, et al. Chongsuphajaisiddhi T, et al. Cardiac effects of antimalarial treatment with haolofantrine. Lancet 1993; 341: 1054–6
Castot A, Rapoport P, Le Coz P. Prolonged QT interval with halofantrine. Lancet 1993; 341: 1541–2
Karbwang J, Laothavorn P, Na Bangchang K, et al. Effect of halofantrine on electrocardiographic changes in uncomplicated falciparum malaria patients. Lancet 1993; 342: 8869
Ketrangsee S, Vijaykadga S, Yamokgul P, et al. Comparative trial on the response of Plasmodium falciparum to halofantrine and mefloquine in Trat Province, Eastern Thailand. Southeast Asian J Trop Med Public Health 1992; 23: 55–8
Bernard J, Sarrouy J, Dupasquier I, et al. Treatment of imported Plasmodium falciparum malaria by halofantrine: 59 cases treated. Med Trop 1990; 50: 167–71
Braendli B, Loutan L, Markwalder K, et al. Treatment of acute malaria with halo-fantrine in Switzerland-preliminary results of a clinical study. 17th International Congress of Chemotherapy; Berlin, 1991
Maisonneuve H, Joly F, John M, et al. Effectiveness of halofantrine in Plasmodium falciparum and Plasmodium vivax malaria in a resistance area (French Guiana). Pressee Med 1988; 17: 99–102
Mashako MNL, Kingway MP, Kayembe N. Treatment of falciparum malaria with halofantrine hydrochloride in a drug resistant region. Analysis of 54 pediatric cases. Ann Soc Belge Med Trop 1990; 70: 25–32
Richard-Lenoble D, Kombila M, Martz M, et al. Efficacy, safety and acceptability of halofantrine in the treatment of acute Plasmodium falciparum malaria in African children (Gabon). Parasitology Today 1989; (Suppl.): 59–63
Salako LA, Sowunmi A, Walker O. Evaluation of the clinical efficacy and safety of halofantrine in falciparum malaria in Ibadan, Nigeria. Trans R Soc Trop Med Hyg 1990; 84: 644–7
Watkins WM, Lury JD, Kariuki D, et al. Efficacy of multiple-dose halofan-trine in treatment of chloroquine-resistant falciparum malaria in children in Kenya. Lancet 1988; 2: 247–50
Coulaud JP, Le Sras J, Matheron S, et al. Treatment of imported cases of falciparum malaria in France with halofantrine. Trans R Soc Trop Med Hyg 1986; 80: 615–6
Cosgriff TM, Boudreau EF, Pamplin CL, et al. Evaluation of the antiamalrial activity of the phenantrine metahnol halofantrine (WR 171,669). Am J Trop Med Hyg 1982; 31: 1075–9
Wirima J, Khoromana C, Molyneux ME, et al. Clinical trials with halofantrine hydrochloride in Malawi. Lancet 1988; 340: 250–1
Adagu SI, Lege-Oguntoye L, Ukoyah JN, et al. Preliminary results of investigations on the in vivo/in vitro sensitivity of P.falciparum to halofantrine in Zaria, Northern Nigeria. Eur J Pharmacol 1990; 183: 1025–6
Chitchang S, Wongteptien S. A clinical trial of halofantrine in acute uncomplicated malaria in Thai soldiers. Parasitology Today 1989; (Suppl.): 21–6
Karbwang J, Milton KA, Na Bangchang K, et al. Pharmacokinetics of halofantrine in Thai patients with acute uncomplicated falciparum malaria. Br J Clin Pharmacol 1991; 31: 484–7
Rab SM, Saeed Sheikhani M, Mahmoud SA, et al. The efficacy of halofantrine hydrochloride in acute malaria: a study of 74 patients from Karachi, Pakistan. Parasitology Today 1989; (Suppl.): 36–44
Roue R, Debord T. Preliminary results. Efficacy, safety of halofantrine in the treatment of a first Plasmodium falciparum malaria attack in adults. Abstract TuP-1-6. Excerpta Medica International Congress Series no. 1988; 810: 129
Weinke T, Loscher T, Fleischer K, et al. The efficacy of halofantrine in the treatment of acute malaria in nonimmune travellers. Am J Trop Med Hyg 1992; 47(1): 1–5
Clernix J, Taelman H. Halofantrine treatment of uncomplicated malaria with high parasitaemia. Trans R Soc Trop Med Hyg 1993; 87: 80
Gay F, Bustos DG, Diquet B, et al. Cross resistance between mefloquine and halofantrine. Lancet 1990; 2: 1262
Shanks GD, Watt G, Edstein MD, et al. Halofantrine given with food for falciparum malaria. Trans R Soc Trop Med Hyg 1992; 86: 233–4
Karbwang J, Na Bangchang K, Thimasarn K, et al. Mefloquine levels in patients with mefloquine resistant Plasmodium falciparum in the eastern part of Thailand. Southeast Asian J Trop Med Public Health 1993; 24(2): 226–9
Fadat G, Louis FJ, Louis JP, et al. Efficacy of micronized halofantrine in semi-immune patients with acute uncomplicated falciparum malaria in Cameroon. Antimicrob Agents Chemother 1993; 37(9): 1955–7
Gillespie SH, Msaki EP, Ramsay A, et al. A new micronized formulation of halofantrine in the treatment of acute Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg 1993; 87: 467–9
Boudreau EF, Canfield CJ, Horton RJ. Halofantrine: an overview of its use in children. Am J Trop Med Hyg 1991; 45 (Suppl.): 272
Fleckenstein L, Pamplin CL, von Bredow J, et al. Comparative phar-macokinetics (PK) of mew antimalarials. Abstract B49. Clin Pharmacol Ther 1983; 33: 234
Flaharty K, Broom C, Eagle S, et al. Pharmacokinetics of commercial halofantrine tablets versus the commercial suspension in healthy volunteers [abstract]. Pharm Res 1991; 8 Suppl. 10: S298
Karbwang J, Ward SA, Milton KA, et al. Pharmacokinetics of halofantrine in healthy Thai volunteers. Br J Clin Pharmacol 1991; 32: 639–40
Krishna S, ter Kuile F, Supanaranond W, et al. Pharmacokinetics, efficacy and toxicity of parenteral halofantrine in uncomplicated malaria. Br J Clin Pharmac 1993; 36: 585–91
Wareham K, Shepard T, Lee RM, et al. SK&F Report on Protocol 102886/A01/UK: Report of a study in healthy male subjects to assess the pharmacokinetics and dose-proportionality of single oral doses of halofantrine (250 to 1000 mg), 1986
Wareham K, Broom C, Wood P, et al. SK&F Report: A study in healthy male subjects to investigate the effect of multiple dosing on blood concentrations of halofantrine, 1988
Veenendaal JR, Parkinson AD, Kere N, et al. Pharmacokinetics of halofantrine and N-desbutylhalofantrine in patients with falciparum malaria following a multiple dose regimen of halofantrine. Eur J Clin Pharmacol 1991; 41: 161–4
Milton KA, Edwards G, Ward SA, et al. Pharmacokinetics of halofantrine in man: effects of food and dose size. Br J Clin Pharmacol 1989; 28: 71–7
WHO. Advances in malaria chemotherapy. Report of a Scientific Group. Technical Report Series 711, 1984
Nothdurft HD, Clemens R, Bock HL, et al. Halofantrine: a new substance for treatment of multidrug-resistant malaria. Clin Invest 1993; 71: 69–73
Basco LK, Gillotin C, Gimenez F, et al. Antimalarial activity in vitro of the N-desbutyl derivative of halofantrine. Trans R Soc Trop Med Hyg 1992; 86: 12–3
Cheng KN, Elsom LF, Hawkins DR. Identification of metabolites of halofantrine, a new candidate anti-malarial drug, by gas chromatography-mass spectrometry. J Chromatogr 1992; 581: 203–11
Basco LK, Gillotin C, Gimenez F, et al. In vitro activity of the enantiomers of mefloquine, halofantrine and enpiroline against Plasmodium falciparum. Br J Clin Pharmacol 1992; 33: 517–20
Shmuklarsky MJ. WRAIR Report: the comparative bioavailability of 3 oral formulations of halofantrine in healthy human subjects. Washington: Walter Reed Army Institute of Research, 1987
Milton KA, Hoaksey PE, Ward SA, et al. Lack of effect of halofantrine on hepatic drug metabolism in the rat in vivo and in vitro. Biochem Pharmacol 1990; 39: 1581–6
Na Bangchang K, Karbwang J, Back DJ. Primaquine metabolism by human liver microsomes: effect of other antimalarial drugs. Biochem Pharmacol 1992; 44: 587–90
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Karbwang, J., Bangchang, K.N. Clinical Pharmacokinetics of Halofantrine. Clin. Pharmacokinet. 27, 104–119 (1994). https://doi.org/10.2165/00003088-199427020-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199427020-00003