Summary
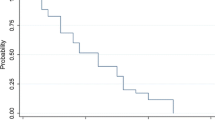
To assess the response rates and toxicity of AZQ in children with recurrent brain and other malignant solid tumors, a phase II study was implemented by the Pediatric Oncology Group. Eligible patients received AZQ 18 mg/M2/week i.v. for 4 doses followed by a 2 week rest period. Each dose was given over four hours (1/3 over the initial 20 minutes). After the first year, the dosage was reduced to 13 mg/M2 due to myelotoxicity resulting in treatment delays. No objective responses were observed in 73 evaluable children with various noncentral nervous system tumors. Of the 91 patients with brain tumors, there were 4 CR's and 2 PR's in patients with astrocytoma, ependymoma, glioblastoma multiforme, oligodendroglioma, brain stem glioma and intracranial yolk sac tumor (median duration, 10 months; range, 2–20+ months). Three of 4 CR's were achieved with a dosage of 18 mg/M2/week. An additional 13 children with brain tumors experienced stable or improved disease (duration, 2–36 + months; median 7.5 months). The principal toxicity was myelosuppression which was cumulative but there were also 3 allergic reactions to AZQ. We conclude that for selected brain tumors, the rates of objective response and stable disease plus the duration of responses support further assessment of AZQ in combination with other agents. Furthermore, the 18 mg/M2 dosage may provide better responses.
Similar content being viewed by others
References
Bender JF, Grillo-Lopez AJ, Posada JG: Diaziquone(AZQ). Invest New Drugs 1:71–84, 1983
Savaraj N, Lu K, Feun LG, Leavens ME, Stewart D, Burgess MA, Benjamin RS, Loo TL: Intracerebral penetration and tissue distribution of 2,5-diaziridinyl 3,6-bis(carboethoxyamino) 1,4-benzoquinone (AZQ, NSC-182986). J Neuro-Oncol 1:15–19, 1983
King CL, Wong S, Loo TL: Alkylation of DNA by the new anticancer agent 3,6-diaziridinyl-2,5-bis(carboethoxyamino) 1,4-benzoquinone (AZQ). Eur J Cancer Clin Oncol 20:261–264, 1984
King CL, Hittelman WN, Loo TL: Induction of DNA strand breaks and cross-links by 2,5-diaziridinyl 3,6-bis(carboethoxyamino) 1,4-benzoquinone in Chinese hamster ovary cells. Cancer Res 44:5634–5637, 1984
Bedikian AY, Bodey GP, Burgess MA, Freireich EJ: Phase I study of aziridinylbenzoquinone. Cancer Clin Trials 4:459–463, 1981
Curt GA, Kelley JA, Kufta CV, Smith BH, Kornblith PL, Young RC, Collins JM: Phase II and pharmacokinetic study of aziridinylbenzoquinone [2,5-diaziridinyl 3,6-bis(carboethoxyamino) 1,4-benzoquinone (AZQ, NSC-182986) in high-grade gliomas. Cancer Res 43:6102–6105, 1983
Haid M, Khandekar JD, Christ M, Johnson CM, Miller SJ, Locker GY, Merrill JM, Reisel H, Hatfield A, Lanzotti V, Stiff P, Shaw J, Krauss S, Showel J, Blough R, Gordon L: Aziridinylbenzoquinone in recurrent, progressive glioma of the central nervous system. A phase II study by the Illinois Cancer Council. Cancer 56:1311–1315, 1985
Feun LG, Yung WA, Leavens ME, Burgess A, Obbens EA, Bedikian AY, Savaraj N, Stewart DJ, Benjamin RS, Fields WS, Bodey GP: A phase II trial of 2,5-diaziridinyl 3,6-bis(carboethoxyamino) 1,4-benzoquinone (AZQ, NSC-182986) in recurrent primary brain tumors. J Neuro-Oncol 2:13–17, 1984
Maral J, Poisson M, Pertuiset BF, Mashaly P, Weil M, Jacquillat CI, Grillo-Lopez AJ: Phase II evaluation of diaziquone (CI-409, AZQ) in the treatment of human malignant glioma. J Neuro-Oncol 3:245–249, 1985
Eagan RT, Dinapoli RP, Cascino TL, Scheithauer B, O'Neill BP, O'Fallon JR: Comprehensive phase II evaluation of Aziridinylbenzoquinone (AZQ, Diaziquone) in recurrent human primary brain tumors. J Neuro-Oncol 5:309–314, 1987
Bjornsson TD, Schold SC, Freidman HS, Schneider D, Falletta JM: Pharmacokinetics of diaziquone after three different dosage regimens. Cancer Treat Rep 69:1383–1385, 1985
Tan CTC, Hancock CH, Mondora A, Hoffman MW: Phase II study of Aziridinylbenzoquinone (AZQ, NSC 182986) in children with cancer. Cancer Res 44:831–835, 1984
Ettinger LJ, Seigel SE, Belasco JB, Evans AE, Ruccione KS, Jamin DC, Rohrbaugh TM, Higgins GR: Phase I clinical evaluation of diaziquone in childhood cancer. Cancer Treat Rep 69:323–327, 1985
Ettinger LJ, Krailo M, Krivit W, Ruccione K, Hammond D: Phase II study AZQ in childhood brain tumors. Proc Amer Soc Clin Oncol 4:238, 1985
Schold SC, Mahaley MS, Vick NA, Friedman HS, Burger PC, DeLong ER, Albright RE, Bullard DE, Khandekar JD, Cairncross JG, Macdonald DR, Falletta JM: Phase II diaziquone-based chemotherapy trials in patients with anaplastic supratentorial astrocytic neoplasms. J Clin Oncol 5:464–471, 1987
Author information
Authors and Affiliations
Additional information
Address for offprints: Pediatric Oncology Group, Operations Office, 4949 West Pine Boulevard, St. Louis, MO 63108, USA
Rights and permissions
About this article
Cite this article
Castleberry, R.P., Ragab, A.H., Steuber, C.P. et al. Aziridinylbenzoquinone (AZQ) in the treatment of recurrent pediatric brain and other malignant solid tumors. Invest New Drugs 8, 401–406 (1990). https://doi.org/10.1007/BF00198601
Issue Date:
DOI: https://doi.org/10.1007/BF00198601