Abstract
The outcomes of COVID-19 in kidney transplant recipients have shown high mortality. In addition to their immunocompromised states, kidney transplant recipients frequently have certain exacerbation risk comorbidities of COVID-19, such as diabetes mellitus, hypertension, and chronic kidney disease. Several concomitant diseases develop during the course of COVID-19, one of which is thromboembolism, which can potentially lead to a critical condition. However, thromboembolic complications in kidney transplant recipients with COVID-19 have not been fully addressed in previous studies. A 62-year-old man, who underwent kidney transplantation 17 years ago, was diagnosed with COVID-19 and was admitted to our hospital. Although the patient was in remission at the start of the hospitalization, his condition became severe on day 7 after admission, with fever, elevated white blood cell counts (10,000/μL) and a high C-reactive protein level (6.9 mg/dL). Although the patient was not under forced bed rest, an ultrasound study on day 10 detected deep venous thrombosis (DVT), with an elevated D-dimer level (6.2 µg/dL). We withdrew the mycophenolate mofetyl and the tacrolimus dosage but did not administer any specific treatment for COVID-19. The patient achieved successful clearance of SARS-CoV-2 on day 16. The DVT disappeared after systematic heparin treatment and oral rivaroxaban for 2 months. DVT occurred in a kidney transplant recipient with COVID-19 who was not bedridden and might manifest when the clinical status was exacerbated during hospitalization.
Similar content being viewed by others
Background
The novel coronavirus disease 2019 (COVID-19) has rapidly spread globally, and its clinical features have gradually been elucidated through numerous reports from all over the world [1,2,3]. A recent study of 72,314 patients in China with mild to severe symptoms reported an overall mortality rate of 2.3% [1]. Another study from Wuhan (China) reported a mortality rate after hospitalization of 4.3% [2]. However, solid organ transplant recipients and immunocompromised patients have much more severe conditions and are thought to be at increased risk of severe outcomes from COVID-19.
Hypertension, diabetes mellitus, and chronic kidney disease have been reported as exacerbation risk factors for COVID-19 [4], and these comorbidities are frequently present in kidney transplant recipients compared with the general population [5, 6]. Kidney transplant recipients with COVID-19 can therefore potentially develop severe conditions compared with COVID-19 patients with no comorbidities. A recent systematic review suggested that COVID-19 has resulted in severe outcomes for kidney transplant recipients. Approximately 30% of hospitalized COVID-19 kidney transplant recipients required care in intensive care units, and 45% of hospitalized kidney transplant recipients developed acute respiratory distress syndrome [7]. More recent reports have suggested that the mortality rate of hospitalized kidney transplant recipients is approximately 30% [8, 9].
The unique feature of COVID-19 is that infected patients experience a variety of concomitant symptoms other than those related to pneumonia, such as dysgeusia, conjunctivitis, and gastrointestinal symptoms [10,11,12]. One of these concomitant diseases is thromboembolism, which sometimes leads to critical conditions [13]. Although a substantial number of incidents related to deep vein thrombosis (DVT) have been reported among the general population with COVID-19 [14], DVT in COVID-19 kidney transplant recipients has not been fully addressed. There have been few reports on thromboembolic complications, not including DVT, in organ transplant recipients [15, 16]. Herein, we experienced a kidney transplant recipient with DVT that developed after hospitalization and reported the clinical time courses in this patient.
Case presentation
A 62-year-old man with end-stage renal disease due to IgA nephropathy received a living-related kidney transplant from his father 17 years earlier. His immunosuppression therapy has included extended-release tacrolimus (TacER, 1 mg daily), mycophenolate mofetil (MMF, 1500 mg daily), and methylprednisolone (4 mg daily). He had not experienced any rejection episodes, and his serum creatinine levels were stable (1.6–1.8 mg/dL). After transplantation, his comorbidities were hypertension and hyperuricemia.
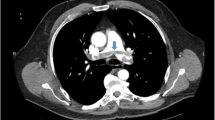
He visited our hospital’s out-patient department with a fever (> 38 °C) that started 2 days ago. He had no concomitant symptoms including respiratory distress symptoms. He had a blood pressure of 108/62 mm Hg, a heart rate of 77 bpm, a respiratory rate of 20 bpm, and an oxygen saturation at room air of 95%. The laboratory data were as follows: white blood cells (WBC), 8100/μL (neutrophils, 7023/μL; lymphocytes 535/μL); C-reactive protein (CRP), 5.6 mg/dL; procalcitonin, 0.05 ng/mL; and IL-6, 6.4 pg/mL. His kidney allograft function was stable (serum urea nitrogen of 17 mg/dL and serum creatinine of 1.52 mg/dL). A computed tomography (CT) scan revealed multiple regions of focal ground-glass opacity (GGO) in both lung fields (Fig. 1 A-C). The possibility of pneumocystis pneumonia was ruled out by the normal range of serum β-D glucan levels, while the possibility of cytomegalovirus was ruled out by a negative finding in the cytomegalovirus antigenemia assay. The patient was diagnosed with COVID-19 by the positive finding in the antigen testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1930 pg/mL).
After the admission, we started him on ceftriaxone (1 g daily) to cover the possibility of bacterial pneumonia, and substituted the intravenous methylprednisolone (20 mg daily) for all- oral maintenance immunosuppressive drugs (TacER, MMF, and oral methylprednisolone), according to our original immunosuppression modification based on the suggestion from the literature [17]. We chose intravenous methylprednisolone instead of dose-escalated oral methylprednisolone because he had nausea and difficulties for taking oral medication on the day of admission. He was admitted into the isolated ward for COVID-19 patients, but he was allowed to go to the bathroom on the ward, not forced to bed rest. We resumed TacER (1 mg daily) on day 2 because his temperature immediately fell below 38 °C but did not require oxygen treatment. On day 5, we replaced intravenous methylprednisolone treatment with oral methylprednisolone therapy (8 mg daily).
On day 7, his temperature increased to over 38.0ºC; however, the laboratory findings deteriorated as follows: WBC, 10,000/μL (neutrophils, 8000/μL; lymphocytes, 1050/μL); CRP, 6.9 mg/dL. A CT scan performed on day 10 revealed that the GGO regions were enlarged in both lung fields (Fig. 1 D-F). We also checked the D-dimer level, which had increased to 6.2 µg/dL from 1.0 µg/mL at the time of the diagnosis. We evaluated the myocardial enzyme levels and electrocardiogram, but both examinations yielded normal findings. Although the patient was not on bed rest, an ultrasonography of the leg blood vessels revealed DVT in both the right soleal and peroneal veins. (Fig. 2 A, C). The patient was therefore started on intravenous heparin (10,000 units daily), according to the guidelines from the National Center for Global Health and Medicine in Japan [18]. However, we gradually increased the dose of heparin up to 15,000 units daily to extend the activated partial thromboplastin time into the target range (50–70 s), according to our protocol (Fig. 3). Although he did not undergo any specific therapy for COVID-19 (e.g., remdesivir, tocilizumab, and antibody administration), successful clearance of SARS-CoV-2 was confirmed on day 16 (Fig. 3). We resumed MMF (1000 mg daily) on day 16. D-dimer levels improved to baseline levels after introducing anticoagulant therapy (Fig. 3). The patient was discharged on day18, and his anticoagulant therapy was switched to rivaroxaban (10 mg daily), which was continued for 2 months. Ultrasonography performed 2 months after the discharge revealed that the thrombus had disappeared from both vessels.
Laboratory data and treatment time courses after admission. A SIRS-Cov2 antigen was examined on days 1 and 16. The bold solid line represents the body temperatures. The dashed line represents the white blood cell counts. The thin mixed line represents the lymphocyte counts. B Time courses of D-dimer and anticoagulant therapies. i.v. intravenous, p.o. per os, TacER extended-release tacrolimus, WBC white blood cells, lymph, lymphocytes, BT body temperature, and US ultrasonography
Discussion and conclusion
In this case, we presumed that the DVT occurred when the patient’s clinical state in this patient was exacerbated. The development of thrombosis during hospitalization is one of the unique features of COVID-19 patients compared with other patients with viral infection. A previous study reported that the rate of COVID-19 patients who experienced thromboembolic complications, including myocardial infarction, ischemic stroke, and venous thromboembolism, were three-fold higher than that of non-COVID-19 patients with thromboembolic complications [14]. In another study, venous thromboembolism occurred in 3.1% of non-ICU-admitted COVID-19 inpatients [19]. However, the incidence of thromboembolic complications including venous thromboembolism in COVID-19 solid organ transplant recipients has been rarely mentioned in the literature, despite a high mortality rate suggested for this immunocompromised cohort. We found three case reports of COVID-19 solid organ transplant recipients with thromboembolic complications, one with acute femoral arterial thrombosis in a lung transplant recipient, two with acute renal infarction in kidney allografts [15, 16, 20]. A cohort study employing a matched non-transplant control group suggested that acute kidney injury (AKI) developed significantly higher in transplant recipients [21]. Although AKI developed due to multifactorial causes in patients with COVID-19, thrombotic events should be one possibility. Indeed, DVT was not intensively focused in many literatures with COVID-19 transplant recipients, but they did not state that DVT was not detected by exhaustively performed examinations. The screening of DVT by ultrasound examination in all hospitalized patients is most likely impossible in this pandemic situation. We cannot say whether transplant recipients with COVID-19 have a tendency for developing DVT or not, because there have been no cohort study regarding DVT exploration, comparing with non-transplant patients.
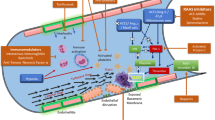
The mechanisms by which DVT developed during disease relapse in this patient are of great concern. The decisive hypercoagulability mechanism in COVID-19 patients is incompletely understood, and several mechanisms such as endothelial injury, hypercoagulability, and complement-mediated endothelial injury have been suggested [22, 23]. Direct SARS-CoV-2 invasion into endothelial cells has been suggested, which might cause microvascular inflammation and endothelial exocytosis, eventually leading to acute respiratory distress syndrome [22]. Indirect endothelial injury has also been suggested, as has the relevance of acute systemic inflammatory mediators such as cytokines (e.g., interleukin-6) [24].
In the present case, the DVT probably formed when systemic inflammation recurred, as evidenced by elevated WBC counts and CRP levels. An in vitro study showed that the SARS-CoV-2 spike protein could activate the alternative complement pathway, which might be another mechanism of indirect endothelial injury [25, 26]. A hypercoagulable state is another suspected etiology of COVID-19-related thrombotic complications, which is defined as the activation of prothrombotic factors such as factor VIII and/or fibrinogen [27, 28]. Although the COVID-19-related hypercoagulable state could meet the criteria of the disseminated intravascular coagulation (DIC) scoring system, its clinical features might differ from those of acute DIC. Thrombosis is the major clinical finding in COVID-19 and is contrasted by bleeding in patients with acute decompensated DIC [28]. However, certain laboratory findings related to the diagnosis of DIC, such as increased D-dimer levels, have also been suggested as a useful monitoring marker for COVID-19 thromboembolic complications [28]. Indeed, elevated D-dimer levels were observed in the present patient, suggesting that some endothelial injury and hypercoagulable change might have occurred in this patient during disease state relapse, although the thrombotic formation was not systemic but rather localized.
In conclusion, we relate the etiology of DVT formation in this kidney transplant recipient to a hypercoagulable state due to severe systemic inflammation due to COVID-19, not due to physical activity restrictions during hospitalization. Given that DVT can develop during COVID-19 reactivation, physicians should consider its possibility if the COVID-19 status relapsed during the hospitalization.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- DVT:
-
Deep vein thrombosis
- TacER:
-
Extended-release tacrolimus
- WBC:
-
White blood cells
- CRP:
-
C-reactive protein
- GGO:
-
Ground-glass opacity
- APTT:
-
Activated partial thromboplastin time
- DOACs:
-
Direct oral anticoagulants
References
Wu Z, McGoogan JM. Characteristics of and Important lessons from the coronavirus disease 2019 (covid-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239–42.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China JAMA. 2020;323:1061–9.
Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–44.
Centers for Disease Control and Prevention. People with Certain Medical Conditions. 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html Accessed 2 Dec, 2020.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52.
Opelz G, Wujciak T, Ritz E. Association of chronic kidney graft failure with recipient blood pressure collaborative transplant study. Kidney Int. 1998;53:217–22.
Marinaki S, Tsiakas S, Korogiannou M, Grigorakos K, Papalois V, Boletis I. A systematic review of covid-19 infection in kidney transplant recipients: a universal effort to preserve patients’ lives and allografts. J Clin Med. 2020;9:2986.
Mamode N, Ahmed Z, Jones G, Banga N, Motallebzadeh R, Tolley H, et al. mortality rates in transplant recipients and transplantation candidates in a high-prevalence covid-19 environment. Transplantation. 2021;105:212–5.
Cravedi P, Mothi SS, Azzi Y, Haverly M, Farouk SS, Pérez-Sáez MJ, et al. COVID-19 and kidney transplantation: results from the TANGO international transplant consortium. Am J Transplant. 2020;20:3140–8.
Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The Prevalence of olfactory and gustatory dysfunction in covid-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020;163:3–11.
Colavita F, Lapa D, Carletti F, Lalle E, Bordi L, Marsella P, et al. SARS-CoV-2 Isolation from ocular secretions of a patient with covid-19 in italy with prolonged viral RNA detection. Annals internal medicine. 2020;173:242–3.
Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, et al. Gastrointestinal manifestations of sars-cov-2 infection and virus load in fecal samples from a hong kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95.
McBane RD, Torres Roldan VD, Niven AS, Pruthi RK, Franco PM, Linderbaum JA, et al. Anticoagulation in COVID-19: a systematic review, meta-analysis, and rapid guidance from mayo Clinic. Mayo Clin Proc. 2020;95:2467–86.
Smilowitz NR, Subashchandran V, Yuriditsky E, Horowitz JM, Reynolds HR, Hochman JS, et al. Thrombosis in hospitalized patients with viral respiratory infections versus COVID-19. Am Heart J. 2020;231:93–5.
Renaud-Picard B, Gallais F, Ohana M, Zeyons F, Kretz B, Andre J, et al. Bilateral acute cardioembolic limb ischemia after coronavirus disease 2019 pneumonia in a lung transplant recipient: a case Report. Transplant Proc. 2020;52:2715–8.
Xu JJ, Samaha D, Mondhe S, Massicotte-Azarniouch D, Knoll G, Ruzicka M. Renal infarct in a COVID-19-positive kidney-pancreas transplant recipient. Am J Transplant. 2020;20:3221–4.
AlOtaibi TM, Gheith OA, Abuelmagd MM, Adel M, Alqallaf AK, Elserwy NA, et al. Better outcome of COVID-19 positive kidney transplant recipients during the unremitting stage with optimized anticoagulation and immunosuppression. Clin Transplant. 2021;35: e14297.
Sato R, Ishikane M, Kinoshita N, Suzuki T, Nakamoto T, Hayakawa K, et al. A new challenge of unfractionated heparin anticoagulation treatment for moderate to severe COVID-19 in Japan. Glob Health Med. 2020;2:190–2.
Hill JB, Garcia D, Crowther M, Savage B, Peress S, Chang K, et al. Frequency of venous thromboembolism in 6513 patients with COVID-19: a retrospective study. Blood Adv. 2020;4:5373–7.
Webb C, Davidson B, Jones ESW, Wearne N, Chetty DR, Blom D, et al. COVID-19-Associated graft loss from renal infarction in a kidney transplant recipient. Kidney Int Rep. 2021;6:1166–9.
Nair V, Jandovitz N, Hirsch JS, Abate M, Satapathy SK, Roth N, et al. An early experience on the effect of solid organ transplant status on hospitalized COVID-19 patients. Am J Transplant. 2021;21:2522–31.
Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–91.
Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–44.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (Lond Eng). 2020;395:1033–4.
Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–9.
Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13.
Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–51.
Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–42.
Acknowledgements
We thank ENAGO for the English language review.
Funding
The authors did not receive any funding.
Author information
Authors and Affiliations
Contributions
HU and KS1 collected the data and wrote the manuscript. AS and KS2 revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
A copy of written consent is available for review by the Editor.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Uematsu, H., Shinoda, K., Saito, A. et al. Deep venous thrombosis in a kidney transplant recipient with COVID-19: a case report. CEN Case Rep 12, 98–103 (2023). https://doi.org/10.1007/s13730-022-00724-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-022-00724-z