Abstract
Background
Melatonin is an endogenous substance which plays a key role in sleep induction by reducing sleep onset latency; it has been approved by the European Food Safety Authority as a food supplement for exogenous administration. Oniria® is a food supplement formulated as 1.98 mg of prolonged-release melatonin tablets; it displays a dual dissolution profile in vitro.
Objectives
The main objective of the present study was to evaluate the relative oral bioavailability of Oniria®, in comparison with immediate-release tablets (IRT) with a similar melatonin content as a reference. We also attempted to characterize the circadian rhythm of endogenous melatonin.
Methods
We performed an open-label, cross-over, randomized, phase I clinical study with two sequences and three periods involving 14 healthy volunteers. We characterized the endogenous melatonin circadian profile (period 1) and pharmacokinetics (PK) of both Oniria® and the reference melatonin (periods 2 and 3).
Results
Two phases were clearly differentiated in the PK profile of Oniria®. An initial one, from dosing up to 2 h, and a delayed one from 2 to 11 h post-administration. During the initial phase, both melatonin formulations were equivalent, with a Cmax value close to 4000 pg/mL. However, in the delayed phase, Oniria® showed significantly higher melatonin concentrations than the IRT (three times higher at 4–6 h post-administration). Moreover, Oniria® exhibited concentrations above the endogenous melatonin peak of 80 pg/mL for up to 2.5 h versus the reference formulation, potentially suggesting an effect of Oniria®, not only in the induction of sleep, but also in the maintenance.
Conclusion
Oniria® could be a highly promising food supplement, not only for sleep induction but also for the maintenance of sleep.
Similar content being viewed by others
This study provides a comparison between the pharmacokinetic profile and oral bioavailability of a melatonin prolonged-release and a melatonin immediate-release formulation. |
Bioequivalence of Oniria® and Melatoplus® were not demonstrated based on the differences found in pharmacokinetic measures (Cmax, AUC). |
Oniria® showed up to three times higher levels of plasma melatonin 4–6 h post-administration. The two-phase release formulation of Oniria enabled melatonin levels to be kept higher than those of peak physiologic melatonin up to 8 h after dosing. |
1 Introduction and Background
Melatonin is an endogenous lipophilic hormone principally produced by the pineal gland [1]. It regulates the circadian rhythm and its synthesis is controlled by the suprachiasmatic nucleus (SCN). Melatonin physiological synthesis is suppressed by light, resulting in low hormone plasma concentrations during the day (< 10 pg/mL). Maximum peak hormone plasma concentration is around 2–4 a.m. (60 pg/mL) [2, 3]. Melatonin therefore plays a decisive role in the physiological control of the circadian sleep–wake cycle [4, 5].
Sleep interruption is known to increase with age; consequently, total sleep time and sleep efficiency undergo a consistent decrease [6]. Moreover, melatonin production diminishes with age—up to 50% in the elderly, in inverse correlation with the frequency of poor sleep quality, and it has therefore been suggested that melatonin deficiency is at least partly responsible for sleeping disorders [7].
Correct sleep is essential for physical and mental health. It is known that poor sleep quality constitutes a risk factor in certain conditions such as obesity, diabetes, heart diseases, depression [8], limited cognitive ability [9,10,11] and lower physical performance.
Endogenous melatonin presents linear kinetics. Following exogenous oral administration of an immediate-release formulation, melatonin half-life ranges from 45 to 65 min. It is rapidly metabolized and is completely eliminated in 3–4 h. In general terms, with a prolonged-release form, a delay in the peak dose can occur; this ranges from 90 to 210 min, depending on the type of preparation. The half-life may be also lengthened, reaching from 3.5 to 4 h [7]. Thus, prototypical prolonged-release forms provide slower and more sustained absorption than the immediate-release ones, the peak dose is delayed and of a lesser magnitude, and levels are maintained from 8 to 10 h, a datum similar to the physiological secretion curve of endogenous melatonin. Thus, this type of prolonged-release melatonin might be mimicking the physiological melatonin secretion curve [2].
Exogenous administration of melatonin as a food supplement was assessed and approved in 2010 by the EFSA (European Food Safety Agency) for the purpose of reducing sleep onset latency, thus favoring induction of sleep [12].
Oniria® is a new melatonin product formulated as prolonged-release oral tablets. Oniria® presents a dual dissolution profile in which approximately 50% is released in the first 20 min (initial phase), and the remaining 50% is maintained for up to 5 h (slower release phase) [13]. The present study attempted to investigate the relative oral bioavailability of this new formulation compared with an immediate-release formulation with a similar melatonin content. As a secondary objective, we characterized the pharmacokinetic profile of endogenous melatonin.
2 Materials and Methods
The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies [14]. Furthermore, the study was conducted and data were processed in accordance with Spanish legislation, the International Council on Harmonization (ICH) guidelines on Good Clinical Practice E6 (R2) [15] and the Revised Declaration of Helsinki [16]. The study protocol was revised and approved by the Research Ethics Committee of the Hospital Universitario de La Princesa on 19 May 2020. All enrolled volunteers signed the informed consent on participating in the study.
2.1 Study Formulations
The test product was melatonin 1.98 mg formulated as prolonged-release tablets; it is marketed by Italfarmaco under the name Oniria®. The reference product was 1.99-mg immediate-release melatonin tablets (IRT), marketed by Lavigor laboratories under the name Melatoplus®.
2.2 Study Population
The study population comprised 14 healthy volunteers enrolled in an investigational study performed at the Clinical Trial Unit of Hospital Universitario de La Princesa (UECHUP).
Prior to inclusion in the study, all subjects were subjected to a thorough examination to establish their suitability to participate in the study.
The volunteers had to meet the following inclusion criteria: men or women aged from 20 to 45 years, body mass index (BMI) of 18.5–25.0 kg/m2 and not diagnosed with relevant clinical conditions or analytical alterations.
Exclusion criteria involved positive drug screening (for cannabis, opiates, cocaine, or amphetamines); the following were also excluded: any pharmacological treatment in the last 15 days, smoking, daily consumption of alcohol and/or acute alcohol poisoning in the last week, having donated blood in the previous month, pregnant or breastfeeding women, history of difficulty swallowing, history of difficulty falling asleep, or non-routine sleep patterns.
2.3 Study Design and Procedures
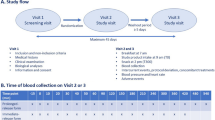
This was an open, randomized, three-period and two-sequence, crossover phase I study; it was conducted under fasting conditions. The study was blinded for analytical determination of the two melatonin formulations.
Washout period was 2 days between each period. Forty-eight hours before starting each period of the study, the volunteers had to refrain from taking drugs, alcohol and any food or drink containing methylxanthines. These restrictions were maintained throughout the time the samples were taken.
During the first period, volunteers were not administered any study formulation. The objective of this initial period involved describing the circadian profile of endogenous melatonin in each participant. In this first period of the study, 26 blood samples (approximately 5 mL each) were collected from each volunteer at the following timepoints: − 12 h, − 11 h, − 10 h, − 9.5 h, − 9 h, − 8.5 h, − 8 h, − 7 h, − 6 h, − 5 h, − 4 h, − 3 h, − 2 h, − 1 h, baseline (in relation to time of product administration in periods 2 and 3), 0.5 h, 1 h, 1.5 h, 2 h, 3 h, 4 h, 5 h, 6 h, 7 h, 9 h, 11 h.
In the other two periods, exogenous melatonin was administered at approximately 10 a.m. and 20 blood samples (approximately 5 mL each) were collected from each volunteer at the following time points: − 2 h, − 1 h (prior to melatonin administration), baseline (4 min before receiving the product), and 0.25 h, 0.5 h, 0.75 h, 1 h, 1.5 h, 2 h, 2.5 h, 3 h, 3.5 h, 4 h, 5 h, 6 h, 7 h, 8 h, 9 h, 10 h and 11 h following administration.
Throughout the three periods, conditions were identical; volunteers remained in a supine position for 17 h (from − 12 to + 5 h post-dose, unless they needed to use the washroom) and with their eyes covered with a mask (from − 12 h to product administration/baseline sample) to protect them from any light exposure. The volunteers were kept in the dark without any source of light.
2.4 Randomization and Administration Process
Treatment was allocated by randomization in balanced blocks of two individuals, with a table of random numbers, to establish a classic cross-over design comprising three periods and two sequences. We assigned to each volunteer a sequence of treatment CTR or CRT being C: Circadian rhythm of endogenous melatonin, T: Melatonin 1.98-mg tablets (Oniria®) and R: IRT 1.99 mg.
In periods 2 and 3, the volunteers fasted for at least 8 h prior to drug administration. The corresponding formulation was administered orally with 240 mL or 8 oz of water. No food or water intake was allowed for at least 5 h after dose administration.
To guarantee treatment compliance, products were dispensed by a member of the research staff.
2.5 Bioanalytical Method
Melatonin concentrations in human plasma samples were measured by means of liquid chromatography coupled to tandem mass spectrometry (triple quadrupole) with an ESI probe in a contract laboratory. The method was previously validated according to European Medicines Agency (EMA) regulations [17]. The limit of quantification was 10 pg/mL; values under this threshold are considered as 0 for descriptive statistics calculation.
2.6 Pharmacokinetic Analysis
We calculated pharmacokinetic parameters employing a non-compartmental analysis with WinNonLin Professional Software (version 8.3, Pharsight Corporation, California, USA).
The primary variables for assessing bioavailability were defined as follows [18,19,20]:
-
AUC0-t Area under the time versus drug concentration curve from 0 to the last observation calculated according to the linear trapezoidal rule.
-
AUC0-inf Area under the time versus drug concentration curve from 0 to infinity.
-
Cmax Observed maximum plasma drug concentration.
We calculated the AUC0–t following the trapezoidal rule. The terminal rate constant (ke) was calculated by linear regression of the log-linear part of the concentration–time curve. The AUC between t and infinity was estimated as Ct/ke (AUCt-inf). The AUC between 0 and ∞ was calculated as AUC0-t + AUCt-inf (AUC0-inf). We determined half-life (t½) was calculated as −ln 2/ke. The remaining pharmacokinetic parameters were directly obtained from the concentration–time curves: maximum concentration (Cmax) and time to reach Cmax (tmax).
For bioavailability assessment, the confidence interval (CI) of 90% for the corresponding mean ratios (test over reference) must be within the predefined bioavailability acceptance range of 80.00–125.00%. According to EMA guidelines, a statistical evaluation of tmax is not required [19].
2.7 Safety Assessment
We compiled adverse events (AEs) spontaneously reported by volunteers or those reported after an open related question. Causality assessment was performed following the algorithm of the Spanish pharmacovigilance system. This algorithm evaluates different criteria such as (a) time and location sequence between drug exposure and AE onset, (b) information on the AE contained in previous literature, (c) drug withdrawal effect and (d) effects following re-exposure to the drug, in order to classify the AE–drug relationship as definite, probable, possible, conditional or unrelated [21].
In addition, all volunteers performed a follow-up visit after completing period 3. In this visit, all volunteers were subjected to a safety analysis (hematology, biochemistry, coagulation and urinalysis) and the female volunteers underwent a pregnancy test. Changes in sleep patterns were not addressed during the study.
3 Results
3.1 Demographic Characteristics
Fourteen healthy volunteers (7 male and 7 female) were enrolled in the study. All volunteers met all the selection criteria, and they all completed the study according to the protocol (Fig. 1).
The volunteers had an average age of 29 years (range 20–45), mean height 1.70 m (range 1.55–1.81), mean weight 63.3 kg (range 52.1–77.3) and mean BMI of 21.8 kg/m2 (range 19.1–24.8) (Table 1). No significant differences were observed between male and female subjects.
3.2 Pharmacokinetics
The means of plasma melatonin concentration reached at each sampling point are shown in Figs. 2 and 3. Endogenous melatonin reached a peak at a plasma concentration of 80 pg/mL around 3 a.m. (Fig. 2), which is in line with data from previous studies [22]. The concentrations reached after either of the two forms of exogenous melatonin administration increased almost 50-fold the endogenous melatonin peak, which was close to 4000 pg/mL as shown in Fig. 3, Panel A. Similarly, Panel A reveals how the pharmacokinetic exogenous melatonin profiles are similar from dosing to 2 h after administration (initial phase). Fig. 3, Panel B shows the different pattern of the two melatonin formulations after reaching maximum concentration, thus demonstrating that Oniria® concentration remains higher at all sampling points from 2 h following product administration (delayed/retarded phase).
Mean plasma melatonin concentrations over time obtained after administration of exogenous melatonin formulations in fasting conditions, compared with the endogenous melatonin profile. A Linear plot of plasma concentrations versus time obtained after administration of melatonin 1.98 mg prolonged-release tablets (Oniria® from Laboratorios ITF Research Pharma S.L.U, test product in fasting conditions), reference melatonin 1.99 mg (immediate-release tablets, Melatoplus® from Laboratorios Lavigor, in fasting conditions) and endogenous melatonin. B Semilogarithmic plot of plasma concentrations versus time obtained after administration of melatonin 1.98 mg prolonged release tablets (Oniria® from Laboratorios ITF Research Pharma S.L.U, test product in fasting conditions), reference melatonin 1.99 mg (immediate-release tablets, Melatoplus® from Laboratorios Lavigor, in fasting conditions) and endogenous melatonin
In the delayed phase, plasma melatonin concentration 4–6 h post-administration exhibited a significant increase with Oniria® compared with IRT (three times higher; Fig. 4). In addition, Oniria® showed concentrations still surpassing the described endogenous melatonin peak of 80 pg/mL 6–8.5 h post-administration (2.5 h longer than the reference formulation) (Fig. 4).
Linear plot of melatonin plasma concentration in the 2–11 h timeframe post-administration (delayed phase). Mean plasma concentrations of exogenous melatonin formulations administered in fasting conditions, compared with endogenous melatonin profile. x3 indicates three times higher Oniria melatonin concentration vs Melatoplus
Table 2 concisely characterizes the kinetic parameters for both exogenous and endogenous melatonins in the whole study group, expressed as arithmetic means (± SD), as well as interindividual variability. Tmax is expressed as median (range). The melatonin primary endpoint means for the bioavailability study provide different results for the two exogenous melatonin formulations, and these profiles are therefore not comparable. These differences are 25.07% in AUC0–t, 25.33% in AUC0–∞ and − 0.57% in Cmax on comparing Oniria® with the reference product. The interindividual variability in Cmax and AUC was similar for both formulations: coefficient of variation of 75.61% for Cmax and 70.99% for AUC0-t for Oniria®; 63.74% for Cmax and 62.62% for AUC0–t for IRT.
The main results of the statistical analysis are summarized in Table 3. Melatonin bioavailability of Oniria® versus IRT melatonin was demonstrated to be different, because the 90% CI for the corresponding mean ratios (test over reference) was not within the predefined bioavailability acceptance range of 80.00–125.00 [18,19,20]. In addition, an exploratory analysis was performed in the partial areas from hour 0 to hour 2 post-administration, where the absorption process takes place; and from hour 2 to 11 post-administration, in which greater availability of Oniria® was observed. Based on a model-independent approach, this mean ratio of Oniria® over IRT was 105.59 for AUC0–2 h (90% CI 90.82–122.77) and 165.92 for AUC2–11 h (90% CI 137.11–200.78). In summary, in the initial absorption phase (up to 2 h post-administration), the bioavailability of both formulations was similar. However, the relative bioavailability for Oniria® from 2 h to 11 h after administration was higher than that of IRT.
In the case of tmax, the median value for melatonin was 0.50 h for Oniria® and 0.75 h for IRT (15 min), with a range of 0.25–1.50 h for both formulations.
The calculated intraindividual coefficients of variation estimated were 19.31% for AUC0–t and 34.15% for Cmax.
3.3 Safety
Only three adverse events were reported in the study (allergic rhinitis, dysmenorrhea and insect bites on back and legs); the causality assessment performed indicated that these were unrelated to the study treatment.
We observed no major changes (to values above 2× upper limit of normal) in the laboratory parameters attributed to any study formulation during the study. The most frequently reported changes involved decreases in erythrocytes, hematocrit or hemoglobin. A clinically negligible decrease of 0.37 g/dL (0.24 g/dL in men and 0.51 g/dL in women) was observed in hemoglobin level, which can be accounted for by the sampling conducted during the study.
4 Discussion
This phase I clinical trial was designed as a single oral dose, open-label, cross-over, randomized study comprising two sequences and three periods to evaluate the pharmacokinetics of two exogenous oral melatonin formulations. These products consisted of prolonged-release melatonin 1.98-mg (Oniria®) and immediate-release melatonin 1.99-mg tablets. Throughout the three periods, 14 healthy volunteers were hospitalized 14 h before product administration time (10:00 a.m.) and released 11 h after the baseline. The endogenous melatonin circadian profile (period 1) and the pharmacokinetics of both melatonin formulations (periods 2 and 3) were evaluated from plasma concentrations.
Regarding endogenous melatonin levels (subjects with an age range of 20–45 years and a mean age of 29 years), a mean plasma peak of 80 pg/mL was reached in the time curve (Fig. 2); these data are in line with previously published studies [22].
The pharmacokinetic profile of Oniria® corresponded to a dual two-phase formulation with an initial phase, observed up to 2 h after administration, of rapid and intense release, followed by a slower and gradual retarded phase up to 6–8 h after administration. These results are consistent with the in vitro dual dissolution profile previously demonstrated for the product [13]. Conversely, the reference product displayed an early, one-phase, shortened release, which tallies better with the behavior of a classic immediate-release formulation [7].
In the exploratory analysis applied to the partial areas, we observed that from time 0 to hour 2, when the absorption process takes place, the bioavailability of both formulations was similar, although tmax for Oniria® was reached 15 min faster than that of the reference melatonin formulation (0.50 h vs 0.75 h, with a range of 0.25–1.50 h for both formulations). In contrast, in the partial area from 2 to 11 h (delayed phase), the relative bioavailability for Oniria® was higher, showing levels above the endogenous melatonin peak for 2.5 additional hours in comparison with the IR melatonin formulation. Additionally, from 4 to 6 h post-administration, Oniria® provided plasma melatonin levels that were three times higher than those produced by IRT.
Considering that both formulations possess a different release profile, they did not prove to be bioequivalent when administered as a single oral dose under fasting conditions. Although during the initial phase the pharmacokinetic profile is consistent with two bioequivalent products, from 2 h post-administration (delayed/retarded phase) the pharmacokinetic profile started to differentiate due to the different release patterns of both melatonin products.
Taking into account the similar melatonin content of both formulations (1.98 vs 1.99 mg), the immediate-release phase included in the dual profile of Oniria® would justify a similar Cmax and tmax in comparison with the IRT. Moreover, the slower release during the following hours might account for the eventual increase in AUC parameters and overall greater bioavailability of Oniria® in relation to the reference melatonin (Table 2). A differential influence of other physicochemical variables, such as the differences between both formulations with regard to particle size, surface area, pH effect or overall dissolution rate cannot be completely ruled out.
In the present study, Oniria® produced levels of melatonin much higher than those achieved by endogenous melatonin; these were maintained for approximately 8 h post-administration. In other words, this appears to indicate that Oniria® can produce melatonin levels clearly higher than the endogenous melatonin plasma peak for as many as 6–8.5 h after administration, thus corresponding theoretically to a regular end-of-sleep period (5 a.m.–7.30 a.m. in case the product is taken at 11 p.m., for instance). Furthermore, according to the data provided by this study, Oniria® would appear to maintain higher plasma melatonin concentrations of over 2.5 h compared with an IRT, and this would suggest improved sleep duration.
In this sense, it is difficult to establish the plasma concentration of melatonin required to induce sleep and, to the best of our knowledge, no studies specify this. Nonetheless, assuming that plasma concentrations above the endogenous melatonin peak are effective with regard to inducing and maintaining sleep, it is hypothesized that Oniria® could be effective not only for sleep induction, but also for maintaining sleep throughout the whole sleeping cycle. It might be of interest to conduct further studies to investigate the effects of Oniria® at both the pharmacodynamic and clinical levels, including its hypnotic effect (induction and maintenance). Despite the fact that there is no clear association between aging and melatonin absorption (although a high degree of interindividual variability [12, 23, 24] has been established), it is also of great interest to evaluate melatonin bioavailability in age groups susceptible to sleep disturbances.
The overall safety analysis of both formulations provided comparable results, a fact that is in accordance with the literature, although no statistical analysis of safety data was performed. All adverse events were resolved by the end of the trial. We detected no clinically significant anomalies in the analytical results, except for a small decrease in the mean hemoglobin value; this has no clinical relevance and can be accounted for by the blood extractions required for the trial. These results concur with those of previous analyses, which demonstrate the absence of any serious side effects following administration of exogenous melatonin, even at doses higher than those of Oniria® and up to 20 mg [25, 26].
5 Conclusion
Our results show that Oniria® presents a two-stage pharmacokinetic profile that exhibits higher plasma melatonin concentrations compared with endogenous melatonin. The release phase of Oniria® is longer lasting than an IR formulation. This renders Oniria® a highly promising food supplement, not only for sleep induction but also for the maintenance of sleep.
References
Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J Am Chem Soc. 1958;80:2587.
Vural EM, van Munster BC, de Rooij SE. Optimal dosages for melatonin supplementation therapy in older adults: a systematic review of current literature. Drugs Aging. 2014;31(6):441–51. https://doi.org/10.1007/s40266-014-0178-0.
Ibrahim MG, Bellomo R, Hart GK, Norman TR, Goldsmith D, Bates S, Egi M. A double-blind placebo-controlled randomised pilot study of nocturnal melatonin in tracheostomised patients. Crit Care Resusc. 2006;8(3):187–91.
Haimov I, Laudon M, Zisapel N, Souroujon M, Nof D, Shlitner A, et al. Sleep disorders and melatonin rhythms in elderly people. BMJ. 1994;309(6948):167. https://doi.org/10.1136/bmj.309.6948.167.
Zeitzer JM, Duffy JF, Lockley SW, Dijk DJ, Czeisler CA. Plasma melatonin rhythms in young and older humans during sleep, sleep deprivation, and wake. Sleep. 2007;30(11):1437–43. https://doi.org/10.1093/sleep/30.11.1437.
Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–73. https://doi.org/10.1093/sleep/27.7.1255.
Poza JJ, Pujol M, Ortega-Albás JJ, Romero O; on behalf of the Insomnia Study Group of the Spanish Sleep Society. Melatonin in sleep disorders. Neurologia (Engl Ed). 2018; vol. 18, p. S0213-4853(18)30200-7. English, Spanish.
Xie Z, Chen F, Li WA, Geng X, Li C, Meng X, et al. A review of sleep disorders and melatonin. Neurol Res. 2017;39(6):559–65. https://doi.org/10.1080/01616412.2017.1315864.
Asayama K, Yamadera H, Ito T, Suzuki H, Kudo Y, Endo S. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. J Nippon Med Sch. 2003;70(4):334–41. https://doi.org/10.1272/jnms.70.334.
Peck JS, LeGoff DB, Ahmed I, Goebert D. Cognitive effects of exogenous melatonin administration in elderly persons: a pilot study. Am J Geriatr Psychiatry. 2004;12(4):432–6.
de Jonghe A, Korevaar JC, van Munster BC, de Rooij SE. Effectiveness of melatonin treatment on circadian rhythm disturbances in dementia. Are there implications for delirium? A systematic review. Int J Geriatr Psychiatry. 2010;25(12):1201–8. https://doi.org/10.1002/gps.2454.
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Scientific Opinion on the substantiation of health claims related to melatonin and alleviation of subjective feelings of jet lag (ID 1953), and reduction of sleep onset latency, and improvement of sleep quality (ID 1953) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2010; 8(2):1467. (14 pp.).
Martínez-Barquero V, González-García J. Dissolution test of Oniria®, a food supplement with melatonin prolonged-release. Biomed J Sci Tech Res. 2020; 25(4):19302–19304.
Tveden-Nyborg P, Bergmann TK, Jessen N, Simonsen U, Lykkesfeldt J. BCPT policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol. 2021;128(1):4–8. https://doi.org/10.1111/bcpt.13492.
European Medicines Agency (EMA) [Internet]. ICH: E 6 (R2): Guideline for good clinical practice - Step 5 [updated 2017 June]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-6-r2-guideline-good-clinical-practice-step-5_en.pdf. Accessed April 2021.
World Medical Association (WMA) [Internet]. WMA Declaration of Helsinki – Ethical principles for medical research involving human subjects [updated 2018 July]. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed April 2021.
European Medicines Agency (EMA) [Internet]. Guideline on bioanalytical method validation. EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2** [updated 2012 February]. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf. Accessed April 2021.
European Medicines Agency (EMA) [Internet]. Guideline on the investigation of bioequivalence. CPMP/QWP/EWP/1401/98 Rev. 1. [updated 2010 August]. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf. Accessed April 2021.
FDA (Food and Drugs Administration) [Internet]. US Department of Health and Human Services. Center for Drug Evaluation and Research (CDER). Guidance for Industry: Bioavalability and bioequivalence studies for orally administered drug products: general considerations [updated 2002 July]. https://www.fda.gov/files/drugs/published/Guidance-for-Industry-Bioavailability-and-Bioequivalence-Studies-for-Orally-Administered-Drug-Products---General-Considerations.PDF. Accessed April 2021.
FDA (Food and Drugs Administration) [Internet]. US Department of Health and Human Services. Center for Drug Evaluation and Research (CDER). Statistical approaches to establishing bioequivalence. Guidance for Industry [updated 2001 January]. https://www.fda.gov/media/70958/download. Accessed April 2021.
Aguirre C, García M. Evaluación de la causalidad en las comunicaciones de reacciones adversas a medicamentos. Algoritmo del Sistema Espanol de Farmacovigilancia [Causality assessment in reports on adverse drug reactions. Algorithm of Spanish pharmacovigilance system]. Med Clin (Barc). 2016;147(10):461–4.
Zhdanova IV, Wurtman RJ, Balcioglu A, Kartashov AI, Lynch HJ. Endogenous melatonin levels and the fate of exogenous melatonin: age effects. J Gerontol A Biol Sci Med Sci. 1998;53(4):B293–8.
Gooneratne NS, Edwards AY, Zhou C, Cuellar N, Grandner MA, Barrett JS. Melatonin pharmacokinetics following two different oral surge-sustained release doses in older adults. J Pineal Res. 2012;52(4):437–45.
Andersen LPH, Werner MU, Rosenkilde MM, Harpsoe NG, Fuglsang H, Rosenberg J et al. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol Toxicol. 2016;17:8. https://doi.org/10.1186/s40360-016-0052-2.
Andersen LPH, Gogenur I, Rosenberg J, Reiter RJ. The safety of melatonin in humans. Clin Drug Investig. 2016;36(3):169–75.
Souza Palmer AC, Zortea M, Souza A, Santos V, Villanova Biazús J, Torres ILS, et al. Clinical impact of melatonin on breast cancer patients undergoing chemotherapy; effects on cognition, sleep and depressive symptoms: a randomized, double-blind, placebo-controlled trial. PLoS ONE. 2020;15(4): e0231379.
Acknowledgements
The authors wish to express their gratitude to the volunteers and to the staff of the Clinical Trial Unit of the Hospital Universitario de La Princesa. Likewise, authors would like to thank Cormac de Brun for the scientific and language editing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by the sponsor ITF Research Pharma S.L.U.
Conflict of interest
F. Abad-Santos, D. Ochoa and M. Román have been consultants or investigators in clinical trials sponsored by the following pharmaceutical companies: Abbott, Alter, Aptatargets, Chemo, FAES, Farmalíder, Ferrer, Galenicum, GlaxoSmithKline, Gilead, Italfarmaco, Janssen-Cilag, Kern, Normon, Novartis, Servier, Teva and Zambon. E. García Aguliar, J. González García, P. Saz-Leal and C. Nieto Magro are employees of ITF Research Pharma S.L.U. The rest of the authors declare that they have no conflicts of interest.
Ethical approval
The study was reviewed and approved by the Research Ethics Committee of the Hospital Universitario de La Princesa in Madrid on 13 May 2020.
Availability of data and material
Qualified researchers or institutions may request data that support the findings of this study from the corresponding author, and the study sponsor.
Code availability
Not applicable.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Authors’ contributions
MR, DO, SMV, and SL-B were involved in study design and supervised the work. PC-M, GM-A, LG-C and AdM-C participated in data collection and sample processing. MR and DO analyzed experimental data. PS-L, FA-S, CNM, DO, EGA and SMV contributed to data interpretation and to the writing of the manuscript. All authors contributed to the interpretation of study data and critically reviewed and approved the final version of the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Román Martinez, M., García Aguilar, E., Martin Vílchez, S. et al. Bioavailability of Oniria®, a Melatonin Prolonged-Release Formulation, Versus Immediate-Release Melatonin in Healthy Volunteers. Drugs R D 22, 235–243 (2022). https://doi.org/10.1007/s40268-022-00394-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-022-00394-3