Abstract
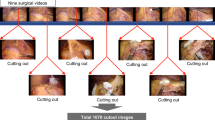
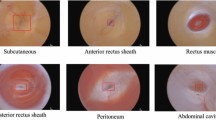
During laparoscopic surgery, surgical gauze is usually inserted into the body cavity to help achieve hemostasis. Retention of surgical gauze in the body cavity may necessitate reoperation and increase surgical risk. Using deep learning technology, this study aimed to propose a neural network model for gauze detection from the surgical video to record the presence of the gauze. The model was trained by the training group using YOLO (You Only Look Once)v5x6, then applied to the testing group. Positive predicted value (PPV), sensitivity, and mean average precision (mAP) were calculated. Furthermore, a timeline of gauze presence in the video was drawn by the model as well as human annotation to evaluate the accuracy. After the model was well-trained, the PPV, sensitivity, and mAP in the testing group were 0.920, 0.828, and 0.881, respectively. The inference time was 11.3 ms per image. The average accuracy of the model adding a marking and filtering process was 0.899. In conclusion, surgical gauze can be successfully detected using deep learning in the surgical video. Our model provided a fast detection of surgical gauze, allowing further real-time gauze tracing in laparoscopic surgery that may help surgeons recall the location of the missing gauze.





Similar content being viewed by others
Abbreviations
- RFID:
-
Radio frequency identification
- AI:
-
Artificial intelligence
- CNN:
-
Convolutional neural network
- mAP:
-
Mean average precision
- IoU:
-
Intersection over Union
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic
- PR:
-
Precision-recall
- AP:
-
Average precision
- FP:
-
False positive
- TP:
-
True positive
- TN:
-
True negative
- CIoU:
-
Complete intersection over union
- PET:
-
Positron emission tomography
- fMRI:
-
Functional magnetic resonance imaging
- CT:
-
Computed tomography
- LBP:
-
Local binary patterns
- RPN:
-
Region proposal network
- GAN:
-
Generative adversarial network
References
Ahmed, K. R. Smart pothole detection using deep learning based on dilated convolution. Sensors (Basel). 21:8406, 2021
Bochkovskiy, A., C. Y. Wang and H. Y. M. Liao. YOLOv4: Optimal Speed and Accuracy of Object Detection. https://arxiv.org/2004.10934v1, 2020.
Chilamkurthy, S., R. Ghosh, S. Tanamala, M. Biviji, N. G. Campeau, V. K. Venugopal, V. Mahajan, P. Rao, and P. Warier. Deep learning algorithms for detection of critical findings in head CT scans: a retrospective study. Lancet. 392:2388–2396, 2018
de la Fuente, E., F. M. Trespaderne, L. Santos, J. C. Fraile and J. P. Turiel. Parallel computing for real time gauze detection in laparoscopy images. In: 2nd International Conference on Bio-engineering for Smart Technologies (BioSMART). https://doi.org/10.1109/BIOSMART.2017.8095328.
de la Fuente López, E., Á. Muñoz García, L. S. Santos Del Blanco, J. C. Fraile Marinero and J. Pérez Turiel. Automatic gauze tracking in laparoscopic surgery using image texture analysis. Comput. Methods Programs Biomed. 190:105378, 2020.
Ding, Y., J. H. Sohn, M. G. Kawczynski, H. Trivedi, R. Harnish, N. W. Jenkins, D. Lituiev, T. P. Copeland, M. S. Aboian, C. M. Mari Aparici, S. C. Behr, R. R. Flavell, S. Y. Huang, K. A. Zalocusky, L. Nardo, Y. Seo, R. A. Hawkins, M. Hernandez Pampaloni, D. Hadley, and B. L. Franc. A deep learning model to predict a diagnosis of Alzheimer disease by using 18F-FDG PET of the Brain. Radiology. 290:456–464, 2019
Dong, X., Y. Lei, T. Wang, M. Thomas, L. Tang, W. J. Curran, T. Liu, and X. Yang. Automatic multiorgan segmentation in thorax CT images using U-net-GAN. Med. Phys. 46:2157–2168, 2019
Everingham, M. and J. Winn. The Pascal Visual Object Classes Challenge (VOC2010) Development Kit.
Ghesu, F. C., E. Krubasik, B. Georgescu, V. Singh, Y. Zheng, J. Hornegger, and D. Comaniciu. Marginal space deep learning: efficient architecture for volumetric image parsing. IEEE Trans. Med. Imaging. 35:1217–1228, 2016
Gibbs, V. C. Retained surgical items and minimally invasive surgery. World J. Surg. 35:1532–1539, 2011
Hashimoto, D. A., G. Rosman, E. R. Witkowski, C. Stafford, A. J. Navarette-Welton, D. W. Rattner, K. D. Lillemoe, D. L. Rus, and O. R. Meireles. Computer vision analysis of intraoperative video: automated recognition of operative steps in laparoscopic sleeve gastrectomy. Ann. Surg. 270:414–421, 2019
Kitaguchi, D., N. Takeshita, H. Matsuzaki, H. Takano, Y. Owada, T. Enomoto, T. Oda, H. Miura, T. Yamanashi, M. Watanabe, D. Sato, Y. Sugomori, S. Hara, and M. Ito. Real-time automatic surgical phase recognition in laparoscopic sigmoidectomy using the convolutional neural network-based deep learning approach. Surg. Endosc. 34:4924–4931, 2020
Lekadir, K., A. Galimzianova, A. Betriu, M. Del Mar Vila, L. Igual, D. L. Rubin, E. Fernandez, P. Radeva, and S. Napel. A convolutional neural network for automatic characterization of plaque composition in carotid ultrasound. IEEE J. Biomed. Health Inform. 21:48–55, 2017
Liu, F., H. Jang, R. Kijowski, T. Bradshaw, and A. B. McMillan. Deep learning MR imaging-based attenuation correction for PET/MR imaging. Radiology. 286:676–684, 2018
Ma, J., F. Wu, T. Jiang, J. Zhu, and D. Kong. Cascade convolutional neural networks for automatic detection of thyroid nodules in ultrasound images. Med. Phys. 44:1678–1691, 2017
Madad Zadeh, S. M., T. Francois, L. Calvet, P. Chauvet, M. Canis, A. Bartoli, and N. Bourdel. SurgAI: deep learning for computerized laparoscopic image understanding in gynaecology. Surg. Endosc. 34:5377–5383, 2020
Nema, S., A. Dudhane, S. Murala, and S. Naidu. RescueNet: an unpaired GAN for brain tumor segmentation. Biomed. Signal Process. Control. 55:101641, 2020
Rahman, R., Z. B. Bin Azad and M. Bakhtiar Hasan. Densely populated traffic detection using YOLOv5 and non-maximum suppression ensembling. Lecture Notes on Data Engineering and Communications Technologies. Proceedings of the International Conference on Big Data, IoT, and Machine Learning:567–578. https://doi.org/10.1007/978-981-16-6636-0_43, 2022.
Ravishankar, H., R. Venkataramani, S. Thiruvenkadam, P. Sudhakar, and V. Vaidya. Learning and incorporating shape models for semantic segmentation. Med. Image Comput. Comput. Assist. Interv. 2017. https://doi.org/10.1007/978-3-319-66182-7_24
Redmon, J., S. Divvala, R. Girshick and A. Farhadi. You only look once: unified, real-time object detection. https://arxiv.org/1506.02640v5, 2015.
Redmon, J. and A. Farhadi. YOLO 9000: Better, Faster, Stronger. https://arxiv.org/1612.08242v1, 2016.
Redmon, J. and A. Farhadi. YOLOv3: an Incremental Improvement. https://arxiv.org/1804.02767v1, 2018.
Ren, S., K. He, R. Girshick, and J. Sun. Faster R-CNN: Towards real-time object detection with region proposal networks. https://arxiv.org/1506.01497, 2015.
Rivera, N., R. Mountain, L. Assumpcao, A. A. Williams, A. B. Cooper, D. L. Lewis, R. C. Benson, J. A. Miragliotta, M. Marohn, and R. H. Taylor. ASSIST - Automated system for surgical instrument and sponge tracking. In: 2008 IEEE Int. Con. on RFID, pp. 297–302, 2008.
Rodriguez-Diaz, E., G. Baffy, W. K. Lo, H. Mashimo, G. Vidyarthi, S. S. Mohapatra, and S. K. Singh. Real-time artificial intelligence-based histologic classification of colorectal polyps with augmented visualization. Gastrointest. Endosc. 93:662–670, 2021
Serra, J., X. Matias-Guiu, R. Calabuig, P. Garcia, F. J. Sancho, and J. P. La Calle. Surgical gauze pseudotumor. Am. J. Surg. 155:235–237, 1988
Shvets, A. A., A. Rakhlin, A. A. Kalinin and V. I. Iglovikov. Automatic instrument segmentation in robot-assisted surgery using deep learning. In: 17th IEEE International Conference on Machine Learning and Applications (ICMLA). https://doi.org/10.1109/ICMLA.2018.00100.
Sombune, P., P. Phienphanich, S. Phuechpanpaisal, S. Muengtaweepongsa, A. Ruamthanthong and C. Tantibundhit. Automated embolic signal detection using deep convolutional neural network. In: Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., pp. 3365–3368, 2017.
Suk, H. I., C. Y. Wee, S. W. Lee, and D. Shen. State-space model with deep learning for functional dynamics estimation in resting-state fMRI. Neuroimage. 129:292–307, 2016
Tokuyasu, T., Y. Iwashita, Y. Matsunobu, T. Kamiyama, M. Ishikake, S. Sakaguchi, K. Ebe, K. Tada, Y. Endo, T. Etoh, M. Nakashima, and M. Inomata. Development of an artificial intelligence system using deep learning to indicate anatomical landmarks during laparoscopic cholecystectomy. Surg. Endosc. 35:1651–1658, 2021
Twinanda, A. P., S. Shehata, D. Mutter, J. Marescaux, M. de Mathelin, and N. Padoy. EndoNet: a deep architecture for recognition tasks on laparoscopic videos. IEEE Trans. Med. Imaging. 36:86–97, 2017
Ultralytics. Yolov5. https://github.com/ultralytics/yolov5, 2020.
Voigtlaender, P., M. Krause, A. Osep, J. Luiten, B. Balachandar Gnana Sekar, A. Geiger and B. Leibe. MOTS: Multi-object Tracking and Segmentation. https://arxiv.org/1902.03604v2.
Yang, X., L. Yu, L. Wu, Y. Wang, D. Ni, J. Qin and P. A. Heng. Fine-grained recurrent neural networks for automatic prostate segmentation in ultrasound images. AAAI. In: Proceedings of the AAAI Conference on Artificial Intelligence 31, 2017.
Zhang, R., Y. Zheng, C. C. Y. Poon, D. Shen, and J. Y. W. Lau. Polyp detection during colonoscopy using a regression-based convolutional neural network with a tracker. Pattern Recognit. 83:209–219, 2018
Zheng, Z., P. Wang, W. Liu, J. Li, R. Ye and D. Ren. Distance-IoU loss: faster and better learning for bounding box regression. AAAI. In: Proceedings of the AAAI Conference on Artificial Intelligence, vol. 34, pp. 12993–13000, 2020.
Acknowledgments
This work was supported by the Ministry of Science and Technology (MOST 110-2634-F-002-009) of the Republic of China (Taiwan) for the financial support in data analysis.
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 90839 kb)
Rights and permissions
About this article
Cite this article
Lai, SL., Chen, CS., Lin, BR. et al. Intraoperative Detection of Surgical Gauze Using Deep Convolutional Neural Network. Ann Biomed Eng 51, 352–362 (2023). https://doi.org/10.1007/s10439-022-03033-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-022-03033-9