Abstract
P2 purinergic receptors are involved in the normal function of the kidney, bladder, and prostate via signaling that occurs in response to extracellular nucleotides. Dysregulation of these receptors is common in pathological states and often associated with disease initiation, progression, or aggressiveness. Indeed, P2 purinergic receptor expression is altered across multiple urologic disorders including chronic kidney disease, polycystic kidney disease, interstitial cystitis, urinary incontinence, overactive bladder syndrome, prostatitis, and benign prostatic hyperplasia. P2 purinergic receptors are likewise indirectly associated with these disorders via receptor-mediated inflammation and pain, a common characteristic across most urologic disorders. Furthermore, select P2 purinergic receptors are overexpressed in urologic cancer including renal cell carcinoma, urothelial carcinoma, and prostate adenocarcinoma, and pre-clinical studies depict P2 purinergic receptors as potential therapeutic targets. Herein, we highlight the compelling evidence for the exploration of P2 purinergic receptors as biomarkers and therapeutic targets in urologic cancers and other urologic disease. Likewise, there is currently optimism for P2 purinergic receptor-targeted therapeutics for the treatment of inflammation and pain associated with urologic diseases. Further exploration of the common pathways linking P2 purinergic receptor dysregulation to urologic disease might ultimately help in gaining new mechanistic insight into disease processes and therapeutic targeting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
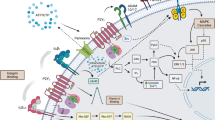
Adenosine triphosphate (ATP) is well-established as the molecular unit of intracellular energy transfer. In the last four decades, ATP has been described in an extracellular context as a signaling molecule via activation of surface membrane P2 purinergic receptors. [1] Since Burnstock’s “purinergic” hypothesis in the early 1970s, the mechanisms that stimulate (mechanical, biochemical, physical, host–pathogen interactions) and mediate (exocytosis, ion channels) ATP release have been well-studied. [1, 2] Briefly, cell injury, necrosis, and apoptosis result in ATP release from intracellular stores via pannexin channels or exocytosis into the extracellular milieu. [1, 3] Extracellular ATP (eATP) activates membrane-bound P2 purinergic receptors of which there are two types: the P2X ionotropic receptors and the P2Y metabotropic receptors (Fig. 1). [1]
P2 purinergic receptor signaling. Extracellular ATP (eATP) is released from injured, necrotic, and apoptotic cells via exocytosis or pannexin channels. Increased concentrations of eATP recruit (e.g., neutrophils) and activate (e.g., macrophages) immune cells. Activation of P2 purinergic receptors increases intracellular calcium initiating signaling cascades that facilitate nociceptive and neuropathic pain, inflammasome assembly and activation, and the induction of proinflammatory cytokines and chemokines
The P2X purinergic receptors are a ligand-gated ion channel-type receptor family of seven mammalian subtypes (P2X1-P2X7) that can form homomeric or heteromeric ion channels. These transmembrane receptors bind eATP in their extracellular loop resulting in a global conformation change that facilitates pore opening through which cations freely move. The typical consequence is Na+-mediated depolarization of the plasma membrane and an increase in the concentration of free cytosolic Ca 2+. [4] The downstream effects include a host of biological responses such as cell proliferation, apoptosis, cell differentiation, immune cell recruitment and activation, and pain transmission that have been studied among different cell types. [1, 3, 4] In general, P2X receptors are ubiquitously expressed, but individual receptors are expressed in varying levels across different cell and tissue types. For instance, P2X4 and P2X7 receptors are predominantly expressed on innate immune cells such as mast cells, macrophages, and neutrophils as they play critical roles during inflammation and immune response against microbes. [1, 3] Alternatively, P2X3 receptor expression is abundant in primary afferent neurons corresponding to its nociceptive function. [5, 6]
The P2Y receptors are a family of G protein-coupled receptors of which eight mammalian subtypes have been identified. [7] Two subfamilies are described as the Gq-coupled P2Y1-like (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11) and the Gi-coupled P2Y12-like (P2Y12, P2Y13, P2Y14) receptors. [7] Unlike their P2X counterparts, P2Y receptors are differentially activated by diverse purines and pyrimidines. [7] When activated, P2Y receptors can couple through the inositol phosphate (IP3) pathway thereby mobilizing Ca [2]+ from intracellular stores that directly control cell function (Fig. 1). [7] Similar to P2X receptors, P2Y receptors are differentially expressed across cell types and can sometimes only be detected in pathological conditions (Table 1). [1] For instance, P2Y6 receptor is highly expressed in T cells infiltrating active inflammatory bowel disease, but is absent in T cells in the unaffected bowel. [8] Diabetes, Alzheimer’s disease, and cancer are likewise among the pathological conditions that induce P2 purinergic receptor expression. [9,10,11]
P2 purinergic receptors have diverse functional roles across multiple cell types, including cell proliferation, apoptosis, cell differentiation, immune cell activation and recruitment, and pain transmission. [1] We focus this review on the expression and function of these receptors in the following urologic diseases: chronic kidney disease (CKD), polycystic kidney disease (PKD), kidney cancer, interstitial cystitis (IC), urinary incontinence, overactive bladder syndrome, bladder cancer, prostatitis, benign prostatic hyperplasia (BPH), prostate cancer, erectile dysfunction, and infertility. Among the most common characteristics of urologic diseases are inflammation and pain.
Inflammation and urologic disease
Inflammation both contributes to and can be the consequence of several urologic diseases. There is substantial evidence that inflammation contributes to renal injury as well as the development and progression of CKD. [12] Urinary tract infections are among the most commonly diagnosed bacterial infections and bladder-related disorders such as interstitial cystitis are likewise strongly associated with inflammation. [13, 14] Chronic inflammation is frequently observed in the adult prostate, is proposed to play a causative role in the development of benign prostatic hyperplasia BPH, and is the defining characteristic of prostatitis. [15,16,17] Chronic inflammation is also a known or suspected risk factor for many cancers including prostate, bladder, and kidney cancers. [17,18,19] Therefore, understanding the mechanisms associated with initiation and propagation of the immune response is essential for improving the management of urologic diseases.
In 1994, Polly Matzinger postulated that the immune system responds to danger signals instead of simply factors that are foreign. [20] While foreign or pathogen-derived signals illicit an immune response, so too could endogenous danger signals released or produced by cells that are injured, stressed, or undergoing cell death. Among the endogenous danger signals is eATP. [21] Considerably greater eATP concentrations are measured in inflamed versus healthy tissues in mouse models of graft-versus-host disease and allergic contact dermatitis. [22] Increased concentrations of eATP act as a powerful chemotactic stimulus for immune cells, many of which express P2 purinergic receptors. [3] Immune cell P2 receptor activation results in chemokine and inflammatory marker induction and the assembly or activation of the inflammasome (Fig. 1).3,21 Propagation of inflammation is thought, in part, to upregulate multiple P2 purinergic receptors on both immune and non-immune cells. [23] Consequently, purinergic signaling continues to be studied in chronic inflammation and in inflammation-associated pathologies such as inflammatory pain, rheumatoid arthritis, and glomerulonephritis. [24]
Pain and urologic disease
Local and referred pain are common symptoms associated with urologic disorders and are often the motivation for seeking medical attention. Infections contribute to a large portion of these concerns; however, other non-infection mediated chronic disorders are also characterized by pain. For instance, interstitial cystitis/bladder pain syndrome (IC/BPS) and chronic nonbacterial prostatitis/chronic pelvic pain syndrome are both diagnosed after clinical infection, malignancy, and/or other identifiable causes for long-term urological pain are ruled out. [13, 15] These disorders can greatly affect the patient’s quality of life. Similarly, advanced genitourinary cancers are characterized by excruciating pain, particularly after bone metastases. [25] Studying pain associated with urologic disease, whether direct or indirect, is important for the holistic treatment of the patient.
Early purinergic signaling studies identified eATP as a neurotransmitter. [2] Evidence that ATP was released from sensory nerves and consequently elicited action potentials in dorsal root ganglionic (DRG) neurons initiated interest in P2 purinergic receptors for their role in pain processing .6 Since then, P2X3 and P2X2/3 receptors were identified as key facilitators of direct neuronal activation (Fig. 1). [6, 26] Activation of other P2 receptors, including P2X4 and P2X7, and some P2Y receptors can modulate pain neurotransmission via indirect mechanisms involving glial-neuronal interactions and/or modulation of other nociceptive-specific receptors. [6] Due to P2 purinergic receptor involvement in various pain states, P2 receptor antagonism has been investigated for pain management in pre-clinical studies and clinical trials. [5, 27] For example, P2X3 antagonist AF-315 produced dose-dependent anti-hyperalgesia in both an adjuvant-induced arthritis rat model and a rat model of knee osteoarthritis. [5]
Kidney
P2 purinergic receptor expression in the kidney has been reported in mice, rats, and in human primary mesangial and visceral glomerular epithelial cells (Table 1). [28,29,30] Purinergic signaling is well-studied in the normal function of the kidney, particularly in blood pressure regulation (Fig. 2). For example, P2Y2 receptors play a prominent role in the regulation of renal electrolyte and water transport. [31] P2Y2 knockout mice have salt-resistant hypertension associated with abnormal renal Na+ and fluid physiology. [32] Furthermore, a P2Y2 receptor agonist reduced blood pressure and increased renal Na+ excretion in mice, indicating that these receptors might be potential therapeutic targets. [33] P2X4 knockout mice are also hypertensive, but dietary Na+ restriction normalizes blood pressure in these mice. [30] Interestingly, a loss-of-function polymorphism in the human P2X4 receptor is associated with increased pulse pressure, but this polymorphism has not been studied specifically in renal function. [34] Altogether, the evidence suggests that P2 purinergic receptors have critical roles in normal kidney function. Here, we discuss how expression and dysregulation of these receptors contribute to kidney disease (Fig. 2).
P2 purinergic receptors in normal kidney function and disease. Activation of P2Y2, P2Y4, and P2X4 contributes to blood pressure maintenance. Ischemia and reperfusion result in increased extracellular nucleotides. Activation of P2X4, P2X7, and P2Y14 initiates signaling cascades that promote chemokine induction, renal tubule necrosis and apoptosis, and neutrophil infiltration contributing to acute kidney injury. P2X7 activation contributes to chronic kidney disease pathogenesis via the NLRP3 inflammasome, while P2Y2 is protective against the same disease. P2X7 activation might also affect cystogenesis via the ERK1/2 pathway culminating in polycystic disease, a risk factor for kidney cancer. P2X6 activation increases kidney cancer cell invasiveness via ERK1/2 and MMP9 signaling
Chronic kidney disease
CKD is the most common disease of the kidney and affects about 15% of the adult population in the USA. [35] Diagnosis is defined by substantially reduced glomerular filtration rate (< 60 mL/min/1.73 m [2]) or albuminuria (≥ 30 mg per 24 h) for more than 3 months [36] and is associated with increased cardiovascular and overall mortality. [35] Persistent, low-grade inflammation is considered a hallmark feature of CKD. [12] The NLRP3 inflammasome contributes to CKD development and P2X7 is a known activator of this inflammasome. [21, 37] Accordingly, a role for P2 receptors in the inflammatory aspects of CKD has been investigated. P2X7 knockout mice fed a high-fat diet had attenuated renal function, reduced inflammation, fibrosis, and oxidative stress compared to wild type (WT) animals. [38] In this study, WT mice fed a high-fat diet had significantly increased urinary protein/creatinine and albumin/creatinine, induced glomerular hypertrophy determined by periodic acid Schiff staining, increased glomerular cell apoptosis determined by active caspase-3 (2.5-fold), increased fibronectin and collagen IV staining, increased oxidative stress marked by upregulation of Nox4 expression, and increased NLRP3 and pro-IL-1β mRNA expression compared to WT mice fed a normal-fat diet (all P < 0.001). [38] All of these parameters were attenuated in P2X7 knockout mice fed a high-fat diet, and this finding was attributed to attenuated NLRP3 inflammasome activation in the P2X7 knockout mice. [38] Others used flow cytometry to show that P2X7 mRNA expression is increased in peripheral blood mononuclear cells (PMBCs) from CKD patients (n = 15) compared to healthy volunteers (n = 15) (P < 0.05) and that P2X7 protein expression is increased specifically on B-lymphocyte (P < 0.05) and monocyte (P < 0.001) populations from patients in early-stage CKD. [39] P2X7 receptors are involved in the defective calcium signaling in PMBCs of patients with CKD by facilitating increased intracellular Ca2+ in these cells. [40] Collectively, these data suggest that P2X7 facilitates CKD pathogenesis. Alternatively, studies indicate that the P2Y2 receptor might serve a protective function. Subtotal nephrectomy (SNX) was performed on P2Y2 knockout mice as a model of CKD. P2Y2 knockout mice had reduced survival after SNX compared to after sham surgery (63.1%, n = 19 vs 100%, n = 9; P < 0.01, respectively), while WT mice had no statistical difference in survival rate (88.9% vs 100%). [41] P2Y2 knockout mice also had increased blood pressure (177 ± 2 mmHg) compared to WT mice (156 ± 7 mmHg) at day 56 post-SNX (P < 0.05). [41] Finally, P2Y2 knockout mice exhibited greater overall renal injury evidenced by increased serum urea (P < 0.05) and increased urine albumin-to-creatinine ratio (UACR) (2.5-fold, P < 0.05) after SNX compared to WT mice. [41]
Acute kidney injury (AKI) is associated with CKD and is a leading cause of mortality or morbidity in hospitalized patients. [42, 43] Patients with severe CKD (< 15 mL/min/1.73 m2) had a dramatically increased risk of AKI (odds ratio = 47.17; CI: 39.22–56.74) compared to non-CKD patients (≥ 60 mL/min/1.73 m2). [44] Several meta-analysis studies have associated AKI with later development of CKD. [43, 45] A major cause of AKI is renal ischemia and reperfusion (IR) resulting in renal tubular necrosis, inflammation, and apoptosis. [42] Consequently, increased eATP after IR activates P2X4 or P2X7. Activation of P2X4 results in NLRP3 inflammasome induction in renal proximal tubule cells, which exacerbates ischemic AKI. [46] Activation of P2X7 induces peptidyl arginine deiminases 4 (PAD4), which is known to exacerbate ischemic AKI, via PKC activation in human and mouse renal proximal tubule cells. [42] Excitedly, early P2X7 inhibition protected against IR-induced AKI in a mouse model.[47] Similarly, P2Y14 inhibition or genetic ablation inhibited IR injury–induced chemokine expression and reduced neutrophil renal infiltration, kidney dysfunction, and proximal tubule damage associated with AKI. [48] Concentrations of P2Y14 ligand, UDP-glucose, are elevated in urine samples from patients who develop AKI compared to patients without AKI. [48] These studies identify P2X7 and P2Y14 as potential therapeutic targets for the treatment of AKI.
Polycystic kidney disease
Polycystic kidney disease (PKD) is a genetic disorder that afflicts about 500,000 people in the USA [49] and over 10 million people worldwide. [50] PKD is often characterized by hypertension, pain, hepatic fibrosis, and end-stage renal disease. [50] P2Y2, P2Y6, and P2X7 mRNA expression was increased in a rat model of autosomal dominant PKD (ADPKD), the most common form of the disease, compared to WT littermates. [51] Protein expression of each receptor was confirmed by immunohistochemistry (IHC). [51] In a pkd2 morphant ADPKD zebrafish model, P2X7 mRNA expression was observed earlier and more strongly compared to control embryos. [52] Both P2X7 knockdown and inhibition significantly reduced the frequency of cyst formation via ERK-dependent pathways in pkd2 morphants (55.8%, P < 0.01 and 35.7%, P < 0.01, respectively). [52] Alternatively, Hillman et al. report that P2X7 activation reduced cystogenesis from cell aggregates derived from an autosomal recessive PKD (ARPKD) mouse model. [53] Enhanced ATP release in human ADPKD is reported as well as in mouse and rat ARPKD cell models, compared to controls. [54, 55] Hillman et al. propose that this excess ATP might serve as a natural brake on cyst formation, while others report that P2X7 and other P2 purinergic receptors facilitate cystogenesis. [51,52,53,54, 56] The authors do not discuss this discrepancy. One consideration is the different P2 purinergic profiles observed between ADPKD and ARPKD or across species. For instance, Schwiebert et al. showed that human ADPKD primary cells lack P2Y1 but express P2Y2 and P2Y6 while mouse cpk ARPKD primary cells expressed P2Y1 and P2Y2 but not P2Y6 (Table 2). [54] Further studies are necessary to differentiate P2 purinergic receptor profiles and functionality between ADPKD and ARPKD and to determine which pathways are relevant in human PKD.
Kidney cancer
Renal cell carcinoma (RCC) accounts for about 90% of all kidney cancers. [18] RCC is further classified into subtypes including clear cell (~ 75% of cases), papillary (~ 15%), and chromophobe (~ 5%). [57] Transitional cell carcinoma, Wilms tumor, and renal sarcoma make up the remainder of kidney cancers. Altogether, the 5-year survival rate for kidney and renal pelvis cancer is about 75%. [58] Currently, surgery is the main treatment for kidney cancer and targeted therapies are often adjuvant therapy after surgery or used to treat advanced disease, with minimal demonstrated benefit. [18]
Purinergic signaling has been studied for both causative and prognostic roles in cancer. Substantially greater eATP concentrations are measured in the tumor microenvironment (TME) of mice compared to healthy tissues and receptor overexpression correlates to poorer outcomes in multiple cancer types. [22, 59,60,61,62] In kidney cancer, the average IHC staining score of peritumoral P2X7 expression was significantly higher than intratumoral P2X7 expression (P < 0.001). [62] P2X7 expression was dichotomized into high expression (above median, n = 138) and low expression (below median, n = 135). Kaplan–Meier survival analysis showed that clear-cell renal carcinoma patients with high intratumoral P2X7 expression had worse cancer‐specific survival than those with low intratumoral P2X7 expression (P < 0.001). [62] Furthermore, prognostic accuracy of TNM stage; the University of California Integrated Staging System (UISS); and the stage, size, grade, and necrosis (SSIGN) scoring models was improved when intratumoral P2X7 expression was added. [62] Another study reported that P2X6 activation drives invasiveness of RCC cells via Ca2+-mediated p-ERK1/2/MMP9 signaling. [63]
Interestingly, multiple P2 purinergic receptors are dysregulated in kidney cancer tissues in The Cancer Genome Atlas (TCGA) public gene datasets as assessed via Wanderer. [64,65,66,67,68] For instance, the majority of receptors—P2X1, P2X3, P2X4, P2X7, P2Y1, P2Y4, P2Y6, P2Y13, and P2Y14—were significantly increased in clear cell RCC tumors compared to normal (Fig. 3). Patients with clear cell RCC had the worst survival and a higher expression of immune signature genes in their tumors compared to patients with papillary and chromophobe RCC. [57] Alternatively, the majority of receptors—P2X1, P2X2, P2X5, P2X6, P2Y2, P2Y6, and P2Y14 receptors—were significantly reduced in chromophobe RCC tumors compared to normal (Fig. 3). Interestingly, papillary cell RCC which is described as the heterogenous subtype had a more balanced P2 receptor expression profile with P2X4, P2X7, and P2Y13 receptors increased and P2X2, P2X6, P2Y1, P2Y2, and P2Y14 receptors decreased in tumors compared to normal (Fig. 3). Such profiles may be indicative of the phenotypes observed across the three types of RCC. Future studies are necessary to investigate the association between P2 purinergic receptor expression profiles and kidney cancer phenotypes and outcomes. There is a need to characterize the expression profiles of individual receptor subtypes, determine their function, and assess whether there is any efficacy in targeting these receptors pharmaceutically for the treatment of kidney cancers.
P2 purinergic receptor mRNA expression in TCGA public datasets. P2 purinergic receptor expression is dysregulated in urologic cancers. The heatmap shows significantly increased (yellow) and decreased (blue) P2 purinergic receptor mRNA expression in cancer compared to normal (P < 0.01) as assessed by the Wanderer tool. P2 purinergic receptors were assessed in the urothelial carcinoma (n: normal (N) = 19; tumor (T) = 267), kidney chromophobe (n: normal (N) = 25; tumor (T) = 66), kidney clear cell (n: Normal (N) = 72; tumor (T) = 518), kidney papillary cell (n: normal (N) = 30; tumor (T) = 198), and prostate adenocarcinoma (n: normal (N) = 52; tumor (T) = 374) datasets
Bladder, ureter, and urethra
Release of ATP by urothelium and consequent activation of P2 purinergic receptors are known to be involved in motor and sensory functions of the urinary bladder. [69] Bladder stretch from filling induces cytosolic Ca2+ increase, promoting ATP release from the urothelium (Fig. 4). This eATP binds to P2 purinergic receptors to trigger nerve activation, the sensation of bladder fullness, and the urge to urinate. [69, 70] Purinergic signaling contributes to bladder contraction, voiding reflex, urinary urgency, and related pain. [71] P2X1-7, P2Y1, P2Y2, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14 receptor expressions have each been identified in the human bladder (Table 1). [14, 69, 72,73,74,75,76] Under physiologic conditions, P2 purinergic receptor knockout mice appear to have no substantial differences in bladder function compared to WT. [70]However, under pathological conditions, inhibition of P2 purinergic receptors improves bladder hyperactivity. [70] We discuss the expression and function of P2 purinergic receptors in select bladder-related disorders (Fig. 4).
P2 purinergic receptors in bladder normal function and disease. Urothelial stretch releases ATP which activates P2X receptors to maintain micturition reflex for normal bladder emptying. Activation of P2Y6 results in ATP release, which activates P2X3, increasing voiding frequency and promoting urinary incontinence. Hydrolysis of ATP results in ADP which activates P2Y1 receptors, which decreases voiding frequency. P2Y12 inhibition of adenylyl cyclase results in bladder smooth muscle contraction which increases voiding frequency in overactive bladder syndrome
Interstitial cystitis
IC/BPS is a chronic, painful inflammatory bladder condition characterized by pelvic pain and urinary symptoms without an identifiable cause. [13] IC/BPS is often underdiagnosed and mistreated, in part because the etiology of the disease is not well-understood. Tissue samples from feline IC urothelium showed reduced P2X1 and P2Y2 expression compared to tissues from normal cats. [71] Alternatively in humans, western blotting analysis revealed significant upregulation of P2X1, P2X2, and P2X3 receptor protein expression in urothelial cells isolated from IC patients (n = 8) compared to age-, race-, and sex-matched asymptomatic control subjects (n = 6) (P < 0.05). [77] Similarly, P2Y1, P2Y2, and P2Y11 receptor mRNA expression was significantly upregulated in urothelium in IC patients (P < 0.05). [77] Stretch-activated release of ATP in cultured bladder urothelial cells from IC patients was higher compared to cells from control patients. [77] In another study, P2X2 and P2X3 protein expression was increased in IC patients compared to control patients in spite of the comparable P2X2 and reduced P2X3 mRNA expression in IC patients. [73] The authors propose that potential negative feedback might account for this discrepancy. P2X3 glycosylation in IC was demonstrated, but the functional significance has not yet been determined. [73] Another study used site-directed mutagenesis to determine that glycosylation of P2X3 receptor can be necessary for assembly of the functional trimeric structure and the inability to do so might result in impaired P2X3 function. [78] The field evidently has its challenges with discrepant receptor expression between human tissues and animal models. While these initial studies indicate involvement of P2 purinergic signaling in IC, future studies are necessary to identify a suitable model and ascertain the direct functional consequence of P2 purinergic receptor upregulation.
Urinary incontinence
Urinary incontinence, defined as loss of bladder control or unintentional voiding, is a common condition that is often unreported. Stress incontinence results from a weak or dysfunctional urinary sphincter, urge incontinence results from overactivity of the detrusor muscle, and overflow incontinence results from bladder distention, for instance in men with BPH. [79] Studies in rats demonstrated that activation of P2Y6 increases the voiding frequency of anesthetized rats. [80] The authors also report that P2X3 activation is necessary for P2Y6-related increased voiding frequency while P2Y1 appeared to have an inhibitory effect. Furthermore, P2Y6-induced bladder hyperactivity requires intact bladder nervous circuitry since this effect was not observed in the isolated bladder in vitro. [80] Alternatively, another study found that P2Y6-deficient mice had more frequent micturition, smaller bladder capacity, and shorter bladder contraction duration than WT mice. [76] While the authors attribute this discrepancy to species differences, they also highlight that studies in rats identify P2Y6 in the bladder for its effect on voiding frequency, while the study in mice attributes the increase in voiding frequency to loss of P2Y6 in the CNS, DRG, or both. [76] In humans, P2X3 and P2X5 protein expression was notably absent in detrusors from patients with idiopathic detrusor instability when both receptors are convincingly expressed in detrusors from control patients. [81] Functional roles for P2X3 and P2X5 have not been defined in the context of urinary incontinence.
Overactive bladder syndrome
Overactive bladder syndrome (OAB) is characterized by urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence, in the absence of urinary tract infection or other obvious pathology. [82] Pyuria has been associated with worst OAB symptoms. [14] One study found that there was greater ATP release from urothelium from OAB patients with pyuria than from non-OAB patients or patients with OAB but not pyuria. [14] All P2 purinergic receptors mRNA except P2X4 and P2Y4 were identified in urothelium microdissected from control patients and patients with OAB with or without pyuria (Table 1). Interestingly, compared to control patients, urothelium from OAB patients without pyuria had increased P2Y11 (200-fold) and P2Y13 (tenfold) mRNA expression, while urothelium from OAB patients with pyuria showed significantly increased P2Y2 (100-fold) and P2Y11 (50-fold) mRNA expression (n = 6; P < 0.01). [14] The suggestion is that the presence of bacteria and thereby inflammation elicits increased urothelial ATP release that plays a role in the heightened symptoms associated with pyuric OAB. Patients with OAB can have urodynamic detrusor overactivity (DO), which is the occurrence of involuntary detrusor contractions during bladder filling (phasic DO) or prior to inhibited detrusor contraction voiding at bladder capacity (terminal DO). [74, 82] P2X3 protein levels assessed by western blotting were relatively higher in DO patients than in controls (P = 0.056) and were significantly increased in the phasic DO subgroup compared to controls (n = 58, P ≤ 0.05). [74] P2X3 expression positively correlated with multiple clinical urodynamic parameters including urgency sensation and voided volume in phasic DO patients. [74] P2Y6 protein expression was more prominent in the urothelium of OAB patients compared to their age-matched male controls with BPH but no OAB symptoms. [83] P2Y6 mucosal expression positively correlated with incontinence severity (n = 28, P = 0.009), but not the International Consultation on Incontinence Questionnaire (ICIQ) OAB symptom score. [83] Both ATP and ADP, a P2Y6 agonist, were positively correlated with the ICIQ OAB symptom score, corroborating a role for purinergic signaling and likely P2 purinergic receptors in OAB. [83] P2Y12 KO mice mimic an OAB phenotype, suggestive that P2Y12 agonists might serve as a therapeutic option for OAB while P2Y12 antagonists might be useful for the treatment of bladder underactivity. [84] Further studies are needed to distinguish P2 purinergic receptor involvement in the inflammation associated with OAB and the direct effect on detrusor dysfunction.
Bladder cancer
Bladder cancer is the fourth most common cancer in American men and is less common in women. Fortunately, the incidence rates of bladder cancer have decreased by 1.2% per year over the last decade, but the death rate (4.4 per 100,000) has remained stable. [58] Inflammation is thought to play a role in bladder cancer development, progression, and response to treatment. [19] As previously mentioned, P2 purinergic receptors play a critical sensory role in the normal bladder, and currently relatively little is known about how these receptors act in bladder cancer. One study sought to correlate P2X receptor expression to urothelial differentiation. Western blotting and IHC analysis showed that P2X3 receptor protein expression is similar in normal urothelium (n = 13) and low-grade papillary carcinoma (n = 12), but decreased in high-grade papillary carcinoma (n = 6) of the human bladder. [85] In contrast, P2X5 receptor protein expression was present in normal urothelium and high-grade carcinoma but was diminished in low-grade carcinoma. [85] The authors propose that P2X3 correlates with urothelial differentiation and might be involved in high-grade papillary carcinoma pathogenesis, while P2X5 does not. In another study, P2Y2 enhancer RNA (P2Y2e) was overexpressed in 29 of 38 bladder cancer patient cancer tissues compared to paracancerous tissues. [86] Increased P2Y2e expression was positively correlated to high histological grade, tumor invasion, and late TNM stage. [86] Furthermore, CRISPR knockdown of P2Y2e inhibited cell proliferation, migration, and invasion and induced apoptosis in bladder cancer 5637 and T24 cells. [86] Corroborating these studies, P2Y2 receptor expression was significantly increased in urothelial carcinoma compared to normal (Fig. 3). P2Y6 receptor expression was also increased, but the majority of receptors were downregulated. P2X1, P2X2, P2X6, P2X7, P2Y1, P2Y13, and P2Y14 receptor expression was significantly decreased in tumor compared to normal in urothelial carcinoma datasets (Fig. 3).
Prostate
The prostate is the major male reproductive gland that facilitates mixing of sperm with the other components that make up semen and aid in the ejaculation of complete semen. [87] Across all species, prostate contractility is mediated by both adrenergic and purinergic receptors. [88] One proposed role for P2 purinergic receptors in normal prostate function is in P2X1-mediated contractile response of the prostate (Fig. 5). [89] In their study, White et al. demonstrate impaired nerve-mediated contractions in aged (12 months old) P2X1 knockout mice compared to aged WT mice. [89]
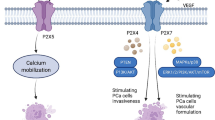
P2 purinergic receptors in prostate normal function and disease. P2X1 receptors are involved in prostate contractility. Activation of P2X3 facilitates pain transmission, while P2X7 activation induces proinflammatory cytokines, both contributing to prostatitis. Activation of P2Y2 promotes prostate cancer cell migration and invasion via the p38, Akt, ERK1/2, MMP3, or MMP13 signaling
Another function of the prostate is likely to serve as a barrier to infection between the bladder and external environment and the remainder of the male reproductive system. [17] The urethra connects the bladder to the penis via the prostate. The testes, epididymis, seminal vesicles, and vas deferens also access the prostate for the passage of semen. Interestingly, prostate cancer is the second most common and bladder cancer the fourth most common cancer in men. [58] Meanwhile, primary cancers of the testicles, epididymis, seminal vesicle, and vas deferens are quite rare. [90,91,92] There is evidence that STIs contribute to prostatic inflammation and the urinary microbiome is thought to influence prostate health. [17, 93, 94] These observations likely contribute to the fact that the prostate is a site of very common diseases, all strongly associated with inflammation and also with aging. P2 purinergic receptors have been identified in prostates from rats, and this expression was reported to increase with aging (Table 1). [95] We review P2 purinergic receptor involvement in prostate diseases (Fig. 5).
Prostatitis
Prostatitis is a heterogeneous disease categorized into four syndromes: (i) acute bacterial prostatitis, (ii) chronic bacterial prostatitis, (iii) chronic prostatitis/chronic pelvic pain syndrome (inflammatory or non-inflammatory), and (iv) asymptomatic inflammatory prostatitis. [96] Although the prevalence of prostatitis is high, reported as high as 9.7% of the US population [96], the precise etiology and mechanism of disease are not always clear. Inflammation and pain are consistent characteristics in men with prostatitis. P2 purinergic receptors have been studied primarily for their nociceptive functions in prostatitis. P2X3 receptors are critical for peripheral pain responses to inflammation and tissue damage. [97] In a rat model of chemically induced prostatic inflammation, P2X3 receptor protein expression was increased in the DRG compared to control groups and a P2X3-specific antagonist attenuated ATP-induced currents in the same region. [98] The authors postulate that prostatic inflammation resulted in increased P2X3 expression, which might account for the neuronal hypersensitivity and subsequent pain associated with prostatitis. Similarly, in a mouse model of prostatitis, the percentage of thoracolumbar DRG neurons expressing P2X2 and P2X3 was significantly elevated with increasing inflammation compared to control mice (n = 4, P < 0.05). [99] In a rat model of chronic prostatitis, increased P2X7 mRNA was detected in the posterior horn of the spinal cord. [100] Inhibition of P2X7 resulted in decreased IL-1β and TNF-α production (P < 0.01) and activation of P2X7 with agonist benzoyl ATP alone was sufficient to significantly increase expression of both cytokines in the spinal cord (P < 0.01 and P < 0.05, respectively). [100] The authors propose that P2 purinergic receptor-mediated inflammation might contribute to the chronic pain associated with prostatitis.
BPH and lower urinary tract symptoms
BPH is described as the progressive enlargement of the prostate gland resulting from nonmalignant proliferation. BPH is prevalent in aging men, with prevalence rates reported from 50 to 75% among men 50 years of age and older to 80% among men 70 years of age and older. [16] White et al. proposes that a P2X1 purinergic contractile response to nerve stimulation develops in the mouse prostate gland with age. [89] This might contribute to the increased muscular tone observed in BPH and highlights P2X1 as a target for the treatment of BPH. [89] BPH can be asymptomatic; however, about half of the men with BPH have lower urinary tract symptoms which include a range of etiologies that are poorly understood. [16] Detrusor underactivity, which leads to incomplete bladder emptying, is one potential symptom. Several studies have identified eATP as necessary for detrusor contractility and increased eATP is associated with increased bladder sensation and voiding frequency. [69, 70, 80, 101, 102] One study identified P2Y6 as a potential channel for ATP release from mucosal urothelium. [72] A selective P2Y6 agonist significantly increased ATP release from stimulated mucosa of control bladder samples (P < 0.05) and this release was significantly attenuated (P < 0.05) by an irreversible P2Y6 antagonist. [72] The authors propose that eATP released via P2Y6 might activate P2X3 and/or P2X2/3 receptors, which in turn release more ATP creating a feedforward loop. In the same study, urothelium/lamina propria (U/LP) strips from patients with BPH (n = 6) released significantly more ATP than U/LP strips from control patients (n = 5) (P < 0.05). [72] Furthermore, they used high performance liquid chromatography experiments to assess ATP catabolism and found a near doubling in the half degradation time of ATP (30 µM) in BPH patients (n = 4) compared to control men (n = 4) (P < 0.05). [72] The combined effects of increase ATP release and decreased ATP degradation might contribute to the high nucleotide levels observed in the bladder mucosa. P2Y6 was identified as the receptor responsible for this release, since P2Y6 agonist PSB0474 increased ATP release and P2Y6 antagonist MRS2578 significantly reduced ATP release in both BPH and control patients (P < 0.05). [72] Immunofluorescence confocal microscopy demonstrated a dramatic reduction in P2X2 and P2X3 protein expression in tissues from patients with BPH compared to control tissues. [72]
Prostate cancer
Prostate cancer is the second leading cause of cancer-related deaths in American men. [58] Chronic inflammation might contribute to prostate carcinogenesis and is associated with aggressive prostate cancer. [17] Phagocytes, including macrophages and neutrophils, respond to inflammatory stimuli by releasing reactive oxygen and nitrogen species that can cause DNA damage, cell injury, and cell death. [17] Consequently, inflammation, cell injury, and cell death result in ATP release and increased eATP concentrations, which can activate P2 receptors on both immune and prostate cells. For instance, P2X4 receptors are involved in neutrophil recruitment, macrophage activation, and differentiation. [103, 104] P2X4, P2X5, P2X7, P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14 receptor expression has been identified in prostate cancer cells (Table 2). [105,106,107,108] P2Y1 activation induces apoptosis and inhibits proliferation of PC3 cells. [105] Activation of P2X5 and/or P2Y11 is proposed to substantially inhibit growth of hormone refractory prostate cancer PC3 and DU145 cells and induce Ca [2]+-independent apoptosis. [109] Alternatively, another study suggests that eATP stimulates DU145 cell proliferation, but the receptor subtype was not identified. [110] The authors propose that hypotonic stress results in ATP release from DU145 cells via intracellular Ca [2]+ signaling, resulting in the activation of ERK1/2, p38, and PI3K, which can facilitate cell proliferation. [110] ATP treatment of PC3 sub-clones, 1E8 (metastatic), and 2B4 (non-metastatic) cells stimulates cell invasion via the Erk1/2 and p38 pathways. [111, 112] ATP treatment alone was also shown to enhance cell motility and cell invasion of DU145, 1E8, and 2B4 cells and altered EMT-related genes, IL-8, Snail, E-cadherin, and Claudin-1. [106, 112] ATP treatment increases the number and length of lamellipodia and filopodia, which are necessary for cell motility as well as their associated Rho GTPases, Rac1 and Cdc42. [112] Furthermore, ATP induces MMP-3 or MMP-13 in DU145, 1E8, and 2B4 cells via AKT and ERK1/2 signaling. [112] P2Y2 siRNA-mediated knockdown identified P2Y2 as one receptor facilitating ATP-induced 1E8 and 2B4 cell migration and invasion. [106] Stable clones of 1E8 P2Y2 shRNA knockdown injected into nude mice did not result in liver metastasis while control cells resulted in liver metastases in 50% of mice. [106] Knockdown of P2Y2 also resulted in reduced tumor growth. [106]
P2X7 receptor protein expression was absent from normal prostate epithelium obtained at autopsy (n = 6) and tissue collected from younger men by transurethral resection (n = 17). [11] Conversely, P2X7 receptor protein expression was detected in 100% of confirmed prostate cancer tissues obtained by biopsy (n = 116), regardless of Gleason score. [11] The authors note that P2X7 expression was detected in earlier biopsies from patients who were eventually diagnosed with prostate cancer, even though the biopsy tissues were considered normal by H&E staining. [11] The authors postulate this finding indicates that P2X7 is a potential diagnostic marker, even in cases where biopsies might initially miss the prostate cancer. In a later study, this group demonstrated that P2X7 expression correlates positively with PSA levels. [113] Specifically, biopsies that were negative for P2X7 were from patients with PSA < 2 ng/mL. [113] The P2X7 described in these studies is described as non-pore functional P2X7 (nfP2X7). It is proposed that high ATP concentrations in the TME drive higher expression of nfP2X7, as demonstrated in vitro. This nfP2X7 was reported as necessary for PC3, DU145, and LNCaP prostate cancer cell survival. [114] Further evidence for P2X7 receptor involvement in prostate cancer is the significant association between the rs3751143 SNP in the P2X7 gene and prostate cancer (odds ratio = 0.86, P = 0.044) in the publicly available American Cancer Genetic Markers of Susceptibility study cohort. [115] The minor allele is a loss-of-function allele and was found to be associated with less aggressive disease (Gleason < 7, n = 484) while the major allele was associate with more aggressive disease (Gleason ≥ 7, n = 688) (odds ratio = 0.77, P = 0.019). [115] In a separate study, survival dimensionality reduction (SDR) analysis revealed genetic interaction profile of P2X7 receptor (rs3751143, rs208294) and VEGFR-2 (rs2071559, rs11133360) polymorphisms with a favorable prognostic profile in prostate cancer patients. [116]
The P2X4 receptor has been identified as a potential therapeutic target for prostate cancer. [108, 117] Our group has demonstrated elevated P2X4 protein (n = 491) and mRNA (n = 120) expression in prostatic intraepithelial neoplasia (PIN) and prostate cancer compared to benign prostate tissues .108 Interestingly, P2X4 receptor protein expression was significantly elevated in prostate cancer cases with PTEN loss (P = 0.0003, n = 389) compared to PTEN intact cases. PTEN is the most commonly inactivated tumor suppressor in prostate cancer and PTEN loss is associated with lethal PCa. [118] PTEN is a negative regulator of PI3K resulting in de-phosphorylation of Akt. [119] Crosstalk between PTEN and PHLPP, another regulator of Akt, promotes prostate cancer cell invasion and is mediated by the P2X4 purinergic receptor. [119] The P2X4 receptor was also shown to be necessary for TGF-β-mediated PC3 cell invasiveness. [119] Cases with PTEN loss commonly exhibit ERG positivity which is observed in about half of prostate cancer cases and typically results from gene fusions between the androgen-regulated gene TMPRSS2 and transcription factor ERG. [120, 121] We also measured elevated P2X4 protein expression in prostate cancer with ERG positivity (P < 0.0001, n = 389) compared to ERG negative cases. [108] Furthermore, P2RY2 is identified among upregulated genes in TMPRSS2:ERG-expressing PC3 cells and was associated with PC3 cell motility and invasiveness. [122] These studies identify mechanistic roles for P2 purinergic receptors in prostate cancer cell motility and invasiveness.
A specific role for P2X4 purinergic receptors in prostate cancer cell motility and invasiveness has been determined. [108, 117] Treatment with the P2X4-specific agonist cytidine 5′-triphosphate (CTP) increased transwell migration and invasion of PC3, DU145, and CWR22Rv1 PCa cells. [108] The P2X4 antagonist5-(3-Bromophenyl)-1,3-dihydro-2HBenzofuro [3, 2-e]-1,4-diazepin-2-one (5-BDBD) resulted in a dose-dependent decrease in viability of PC3, DU145, LNCaP, CWR22Rv1, TRAMP-C2, Myc-CaP, BMPC1, and BMPC2 cells and decreased DU145 cell migration and invasion. [108, 117] Knockdown of P2X4 attenuated growth, migration, and invasion of PCa cells. [108] Knockdown of P2X4 also resulted in significantly attenuated allograft growth in mice [108] and treatment with 5-BDBD delayed PCa xenograft growth in vivo [117].
Prostate cancer most often metastasizes to bone and bone metastases are associated with extreme pain that substantially affects the patient’s quality of life. [25, 123] Multiple P2 purinergic receptors have been implicated in mediating cancer-induced bone pain (CIBP). [124] Specifically, pharmacologic blockade of P2X3 or P2X2/3 has analgesic efficacy in a rat model of CIBP. [27] P2X4 receptor expression in microglia was increased with treatment of chemokine monocyte chemoattractant protein-1 (MCP-1), an established facilitator of CIBP. [125] The authors propose that MCP-1-induced P2X4 expression in microglia via PI3K/Akt signaling can contribute to mechanical allodynia in CIBP. [125] P2X4 mRNA and protein expression were increased in rat models of bone cancer pain and intrathecal injection of P2X4 siRNA attenuated CIBP. [126] The authors concluded that nociceptive hypersensitivity in the CIBP model is dependent on P2X4 receptor signaling in microglia. [126]
The TCGA datasets corroborated elevated P2X4 receptor expression in prostate adenocarcinoma compared to normal (Fig. 3). P2X5 receptor expression was also increased, while P2X1, P2X2, P2X6, P2X7, P2Y2, P2Y13, and P2Y14 receptor expression was decreased (Fig. 3).
Future studies are necessary to characterize the expression profiles of individual receptors, determine their function, and ultimately assess the feasibility of therapeutically targeting P2 purinergic receptors in prostate cancer.
Erectile dysfunction and infertility
Purinergic signaling is important for initiation and maintenance of penile erection. [127] As such, P2 purinergic receptors have been investigated for their role in erectile dysfunction (ED). Rabbit and human cavernosal smooth muscle (CSM) relaxation is mediated by P2Y1, P2Y2, P2Y4, or P2Y6 receptor activation. [127,128,129] Similarly, P2Y1 activation induced contractions in the internal pudental arteries (IPA), the major blood supply to the penis. [130] Meanwhile, P2X1 activation appeared to reduce CSM relaxation and induce IPA and vas deferens contractions in rats. [130,131,132] P2 receptor-mediated CSM relaxation is attenuated in models of diabetes mellitus and bladder outlet obstruction, two conditions that predispose to ED, but is unchanged in rabbit models of chronic renal failure and hypothyroidism, other known risk factors for ED. [127,128,129, 133,134,135] As a proposed explanation for P2 purinergic receptor involvement in ED, a study demonstrated that human corpus cavernosum tissues from men with ED exhibited slower ATP hydrolysis compared to controls resulting in prolonged exposure to endogenous ATP and likely P2 purinergic receptor desensitization. [136] Another study reported reduced P2Y1, P2Y2, P2Y4, and P2Y6 mRNA and protein expression in corpora cavernosum tissues from castrated rats and P2 purinergic receptor expression was positively correlated with serum testosterone levels. [137] Further studies are necessary to deduce whether certain pathologies dysregulate P2 purinergic expression or function to contribute to ED. To date, P2X1 antagonism has been shown to inhibit IPA or vas deferens contraction, P2Y1 antagonism has been shown to inhibit IPA relaxation in rats, and intracavernous injection of P2 purinergic agonist, suramin, improves recovery of ED in a rat model. [130, 132, 138] These data are promising for potential P2 purinergic receptor-based therapeutic options for men with ED.
P2X1-mediated vas deferens contraction has also been studied in the context of fertility. Mice lacking P2X1 have 90% reduced fertility due to reduced sperm in the ejaculate. [139] Evidence suggests that the absence of P2X1 affects sperm transport, but not sperm function in mice. [139, 140] There was also no effect on sexual behavior or function. [140] Consequently, P2X1 has been proposed as a pharmacological target for reversible male contraception, likely in concert with α1A-adrenoceptor antagonism. [140] Other P2 purinergic receptors are thought to contribute to infertility. P2X4 and/or P2X7 protein expression is observed in human testicular peritubular cells and ATP treatment of these cells resulted in pro-inflammatory cytokine production and secretion. [141] These data suggest that P2 purinergic receptors might promote sterile testicular inflammation, which is associated with infertility. ATP activation of peritubular cells via P2 purinergic receptors was shown to drive testicular sperm transport, suggesting a pharmacological target for male infertility and contraception. [142]
Ectonucleotidase control of P2 purinergic receptor agonists in urologic diseases
The regulation of P2 purinergic receptor signaling involves both the modulation of receptor expression as well as the control of agonist availability. Ectonucleotidases are key enzymes that hydrolyze nucleotides, which dictate the duration of activity of P2 purinergic receptor agonists. Of the four major ectonucleotidase families, ecto-nucleoside triphosphate diphosphohydrolases (NTPDases) and ecto-nucleotide pyrophosphatase/phosphodiesterases (NPPs) hydrolyze ATP to ADP and ADP to AMP. [143] The eight NTPDases are generally expressed in all tissues and NTPDase1/CD39, the best characterized ectonucleotidase, is widely expressed on immune cells. [143] Others have reviewed the roles of ectonucleotidases in inflammation, pain, and cancer. [143,144,145,146] Similarly, studies are ongoing to investigate the role of ectonucleotidases in urologic systems. Specifically, Dwyer et al. provide a comprehensive review of ectonucleotidases involved in normal kidney function and renal disease. [147] The authors discuss roles for CD39 in renal inflammation, immunomodulation, acute kidney injury, chronic kidney disease, diabetic nephropathy, polycystic kidney disease, transplantation, and renal cell carcinoma. [147] All eight NTPDases were detected in the mouse urinary bladder with different localizations. [148] Specifically, CD39 was detected primarily in endothelial cells of the detrusor and lamina propia which is consistent with its role in degrading ATP within vasculature aimed at modulating thrombotic events. [148] CD39 also tightly regulates ADP-induced P2Y12-mediated bladder smother muscle contraction. [149] Normal vas deferens contraction is also dependent on CD39. [150] CD39 knockout male mice had reduced fertility due to reduced sperm emission. [150] The authors demonstrated in vitro that P2X1 agonist-mediated contraction was reduced in vas deferens isolated from CD39 knockout mice compared to control mice. [150]
High CD39 protein expression was detected in 43.8% of bladder cancer cases and was associated with non-muscle-invasive phenotype (P < 0.001), and lower tumor stage (P < 0.001). [151] Interestingly, data from multiple datasets via ONCOMINE show that while overexpressed in most solid tumors, CD39 mRNA expression was significantly decreased in bladder and prostate cancers. [146] Another study measured reduced CD39 protein expression on macrovesicles isolated from prostate cancer patients compared to healthy individuals. [152] However, there was increased ATP and ADP hydrolysis in prostate cancer patients compared to healthy individuals, suggesting involvement of alternative ectonucleotidases. [152] The likely candidate is the NPP family, since increased NPP activity was measured in serum and platelets from prostate cancer patients compared to healthy volunteers. [153] Interestingly, there was significantly less ATP hydrolysis in patients with clinical stage IIB and III compared to IIA, suggesting increased availability of eATP in the tumor microenvironment of later stage prostate cancer patients. [152] Altogether, these data suggest that ectonucleotidases are integral in the P2 purinergic receptor signaling associated with tumorigenesis. As such, future investigations on P2 purinergic receptors in urologic cancers must include considerations for ectonucleotidases and their control of P2 purinergic receptor agonists.
Current state of P2 receptor therapies
We have discussed critical roles for P2 purinergic receptors in urologic disorders, highlighting the receptors as potential therapeutic targets. Since 1997, P2Y12 antagonists have been FDA-approved as antiplatelet therapies [154].Subsequently, multiple P2 purinergic receptor agonists and antagonists have entered clinical trials for various indications, including inflammation-related disorders and pain. Specifically, previous and current clinical trials have investigated the P2X3 antagonist gefapixant in treatment of IC/BPS (NCT01569438) [155], pain (NCT01554579) [156], and stress urinary incontinence (NCT04193176) [157]. A phase 2, double-blind, placebo-controlled, randomized study to assess the efficacy of gefapixant in female subjects with moderate to severe pain associated with IC/BPS after 4 weeks of treatment was conducted. Gefapixant-treated patients (n = 36) had a decrease in Numeric Pain Rate Scale (NPRS) score, Painful Bladder/Interstitial Cystitis Symptom Diary (PBIC-SD) score, O’leary-Sant Interstitial Cystitis Symptom Index (ICSI), and Genitourinary Pain Index (GUPI) compared to patients treated with placebo (n = 38) [155]. There were no serious adverse events and only mild adverse events, primarily dysgeusia/hypogeusia [155]. Another phase 2 placebo-controlled, randomized study was conducted to assess the efficacy of a single dose level of gefapixant in subjects with moderate to severe pain associated with osteoarthritis of the knee 4 weeks after treatment. There was also a decrease in the primary outcome measure, NRPS, in gefapixant-treated patients (n = 85) compared to placebo-treated patients (n = 86) [156]. However, there was no improvement in the secondary outcomes, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and walking pain scores [156]. Finally, a phase 3 double-blind, placebo-controlled, randomized study to evaluate the efficacy and safety of gefapixant in improving symptoms of cough-induced stress urinary incontinence (SUI) in adult female participants with refractory or unexplained chronic cough is currently in progress [157]. These trials as well as other pre-clinical studies demonstrate that P2X3 antagonists show tremendous promise in pain management across urologic diseases [5, 97].
P2X7 antagonists are in clinical trials for inflammation-related depression (NCT04116606) [158], rheumatoid arthritis (NCT00628095 [159], NCT00520572 [160]), and inflammatory pain (NCT00849134 [161]). The phase 2 study to evaluate the antidepressant efficacy of P2X7 antagonist JNJ-54175446 was suspended due to the COVID-19 pandemic [158]. Both phase 2 studies evaluating the efficacy of P2X7 antagonists, CE-224,535 [159] and AZD9056 [160], in the treatment of patients with rheumatoid arthritis showed no significant benefit of the drug compared to placebo. Both drugs were well-tolerated by patients [159, 160]. The single-blind, placebo-controlled, randomized phase 1 study was a first-time-in-human trial for the P2X7 antagonist GSK1482160 developed to treat inflammatory pain. Ali et al. used a model-based approach to predict that adequate pharmacological engagement of P2X7 could not be achieved in vivo using safe human exposures [162]. As such, development of the drug was discontinued. The therapeutic relevance of P2X7 receptors in patients with inflammatory pain remains to be tested. New P2X7 antagonists are currently in phase 1 trials to assess safety and tolerability (NCT03151486) [163] (NCT02587819) [164] or P2X7 receptor occupancy (NCT03437590) [165] in healthy individuals, which is exciting for the prospect of P2X7 antagonist therapeutics. As we have reviewed above, there is evidence for P2X3- and P2X7-mediated inflammatory pain in prostatitis [98, 100], which provides rationale for pre-clinical studies to test the efficacy of P2X3 and P2X7 antagonists in this context. Pre-clinical studies are also warranted to investigate targeting P2X7 for the treatment of CKD.
P2X7 receptor expression profiles improved kidney cancer prognostic accuracy as described previously. [62] P2X7 expression is also detected in prostate cancer, but never in benign prostate tissues [11] and a SNP in the P2X7 gene is significantly associated with prostate cancer aggressiveness (P = 0.019). [115] Consequently, P2X7 receptors should be investigated as potential biomarkers in these cancer types. Safety and tolerability studies have been completed for a P2X7 antagonist that has potential as a basal cell carcinoma therapeutic (NCT02587819) [164]. This anti-nf-P2X7 antibody ointment proved safe and well-tolerated, with 65% of patients showing a reduction in lesion area (n = 21) [166].
A P2Y2 agonist, diquafosol tetrasodium ophthalmic solution, has completed phase 3 clinical trials (NCT00403975 [167], NCT00600288 [168], NCT00404131 [169], NCT00037661 [170]) in the USA and is approved in Japan and Korea for the topical treatment of dry eye disease [171]. One study demonstrated that 3% diquafosol may be an effective and safe treatment for the management of dry eye resulting from cataract surgery in patients with preexisting dry eye (NCT02608489) [172]. Another P2Y2 agonist, denufosol tetrasodium, completed phase 3 clinical studies to assess its efficacy in patients with cystic fibrosis (NCT00625612) [173]. While the treatments were well-tolerated, there was no benefit to the treatment group (n = 233) compared to the placebo group (n = 233) with respect to pulmonary function or the incidence of pulmonary exacerbations [174]. As new generation agonists progress from pre-clinical studies, we look forward to studies investigating blood pressure control by managing renal Na+ excretion. Similarly, the blockade of P2X1 showed great promise as a reversible male contraceptive and treatment for ED [140]. However, P2X1 antagonists have not progressed to the clinical testing phase.
Conclusions
P2 purinergic receptors are involved in normal kidney, bladder, and prostate function. Cell injury, necrosis, or apoptosis increase eATP concentrations and, subsequently, P2 purinergic receptor expression. The consequence is receptor dysregulation that might (i) directly disrupt normal urologic functions facilitating pathogenesis, (ii) mediate inflammation associated with urologic diseases, or (iii) facilitate pain associated with urologic diseases. Perhaps all of these might occur simultaneously. While key receptors are identified in some diseases and mechanisms proposed for their function, substantial gaps of understanding remain. Thorough characterization of P2 purinergic receptor expression profiles and function is necessary to bolster the current optimism surrounding therapeutic targeting of P2 purinergic receptors in urologic disease.
Data availability
Not applicable.
References
Burnstock G (2012) Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. BioEssays 34:218–225. https://doi.org/10.1002/bies.201100130
Burnstock G (1972) Purinergic nerves. Pharmacol Rev 24:509
Idzko M, Ferrari D, Eltzschig HK (2014) Nucleotide signalling during inflammation. Nature 509:310–317. https://doi.org/10.1038/nature13085
Samways DSK, Li Z, Egan TM (2014) Principles and properties of ion flow in P2X receptors. Front Cell Neurosci 8:6–6. https://doi.org/10.3389/fncel.2014.00006
Ford AP (2012) In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal 8:3–26. https://doi.org/10.1007/s11302-011-9271-6
Donnelly-Roberts D, McGaraughty S, Shieh C-C, Honore P, Jarvis MF (2008) Painful purinergic receptors. J Pharmacol Exp Ther 324:409–415. https://doi.org/10.1124/jpet.106.105890
von Kügelgen I, Wetter A (2000) Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol 362:310–323. https://doi.org/10.1007/s002100000310
Somers GR, Hammet FM, Trute L, Southey MC, Venter DJ (1998) Expression of the PY purinergic receptor in human T cells infiltrating inflammatory bowel disease. Lab Investig; J Tech Methods Pathol 78:1375–1383
Coutinho-Silva R, Parsons M, Robson T, Lincoln J, Burnstock G (2003) P2X and P2Y purinoceptor expression in pancreas from streptozotocin-diabetic rats. Mol Cell Endocrinol 204:141–154. https://doi.org/10.1016/S0303-7207(03)00003-0
Moore D, Iritani S, Chambers J, Emson P (2000) Immunohistochemical localization of the P2Y1 purinergic receptor in Alzheimer’s disease. NeuroReport 11:3799–3803. https://doi.org/10.1097/00001756-200011270-00041
Slater M, Danieletto S, Gidley-Baird A, Teh LC, Barden JA (2004) Early prostate cancer detected using expression of non-functional cytolytic P2X7 receptors. Histopathology 44:206–215. https://doi.org/10.1111/j.0309-0167.2004.01798.x
Mihai S, Codrici E, Popescu ID, Enciu A-M, Albulescu L, Necula LG, Mambet C, Anton G, Tanase C (2018) Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res 2018:2180373. https://doi.org/10.1155/2018/2180373
Marcu I, Campian EC, Tu FF (2018) Interstitial cystitis/bladder pain syndrome. Sem Reprod Med 36:123–135. https://doi.org/10.1055/s-0038-1676089
Contreras-Sanz A, Krska L, Balachandran AA, Curtiss NL, Khasriya R, Kelley S, Strutt M, Gill HS, Taylor KM, Mansfield KJ, Wu C, Peppiatt-Wildman CM, Malone-Lee J, Duckett J, Wildman SS (2016) Altered urothelial ATP signaling in a major subset of human overactive bladder patients with pyuria. Am J Physiol Renal Physiol 311:F805–F816. https://doi.org/10.1152/ajprenal.00339.2015
Krieger JN, Nyberg J, Leroy and Nickel JC (1999) NIH consensus definition and classification of prostatitis. JAMA 282 236–237 10–1001/pubs.JAMA-ISSN-0098–7484–282–3-jac90006
Egan KB (2016) The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol Clin North Am 43:289–297. https://doi.org/10.1016/j.ucl.2016.04.001
Sfanos KS, Yegnasubramanian S, Nelson WG, De Marzo AM (2018) The inflammatory microenvironment and microbiome in prostate cancer development. Nat Rev Urol 15:11–24. https://doi.org/10.1038/nrurol.2017.167
de Vivar Chevez AR, Finke J, Bukowski R (2014) The role of inflammation in kidney cancer. Adv Exp Med Biol 816:197–234. https://doi.org/10.1007/978-3-0348-0837-8_9
Gakis G (2014) The role of inflammation in bladder cancer. Adv Exp Med Biol 816:183–196. https://doi.org/10.1007/978-3-0348-0837-8_8
Matzinger P (1994) Tolerance, danger, and the extended family. Annu Rev Immunol 12:991–1045. https://doi.org/10.1146/annurev.iy.12.040194.005015
Di Virgilio F (2007) Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol Sci 28:465–472. https://doi.org/10.1016/j.tips.2007.07.002
Falzoni S, Donvito G, Virgilio FD (2013) Detecting adenosine triphosphate in the pericellular space. Interface Focus 3:20120101. https://doi.org/10.1098/rsfs.2012.0101
Burnstock G (2016) P2X ion channel receptors and inflammation. Purinergic Signalling 12:59–67. https://doi.org/10.1007/s11302-015-9493-0
Antonioli L, Blandizzi C, Pacher P, Haskó G (2019) The purinergic system as a pharmacological target for the treatment of immune-mediated inflammatory diseases. Pharmacol Rev 71:345–382. https://doi.org/10.1124/pr.117.014878
Aielli F, Ponzetti M, Rucci N (2019) Bone metastasis pain, from the bench to the bedside. Int J Mol Sci 20(2):280. https://doi.org/10.3390/ijms20020280
Chen C-C, Akopian AN, Sivilottit L, Colquhoun D, Burnstock G, Wood JN (1995) A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377:428–431. https://doi.org/10.1038/377428a0
Kaan TK, Yip PK, Patel S, Davies M, Marchand F, Cockayne DA, Nunn PA, Dickenson AH, Ford AP, Zhong Y, Malcangio M, McMahon SB (2010) Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain: J Neurol 133:2549–2564. https://doi.org/10.1093/brain/awq194
Vonend O, Oberhauser V, Von Kügelgen I, Apel TW, Amann K, Ritz E, Rump LC (2002) ATP release in human kidney cortex and its mitogenic effects in visceral glomerular epithelial cells. Kidney Int 61:1617–1626. https://doi.org/10.1046/j.1523-1755.2002.00315.x
Menzies RI, Booth JWR, Mullins JJ, Bailey MA, Tam FWK, Norman JT, Unwin RJ (2017) Hyperglycemia-induced renal P2X7 receptor activation enhances diabetes-related injury. EBioMedicine 19:73–83. https://doi.org/10.1016/j.ebiom.2017.04.011
Craigie E, Menzies RI, Larsen CK, Jacquillet G, Carrel M, Wildman SS, Loffing J, Leipziger J, Shirley DG, Bailey MA, Unwin RJ (2018) The renal and blood pressure response to low sodium diet in P2X4 receptor knockout mice. Physiol Rep 6:e13899. https://doi.org/10.14814/phy2.13899
Vallon V, Stockand J, Rieg T (2012) P2Y receptors and kidney function. Wiley Interdiscip Rev Memb Trans Signal 1:731–742. https://doi.org/10.1002/wmts.61
Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V (2007) Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. Faseb J 21:3717–3726. https://doi.org/10.1096/fj.07-8807com
Rieg T, Gerasimova M, Boyer JL, Insel PA, Vallon V (2011) P2Y2 receptor activation decreases blood pressure and increases renal Na+ excretion. Am J Physiol Regul Integr Comp Physiol 301:R510–R518. https://doi.org/10.1152/ajpregu.00148.2011
Stokes L, Scurrah K, Ellis Justine A, Cromer Brett A, Skarratt Kristen K, Gu Ben J, Harrap Stephen B, Wiley James S (2011) A loss-of-function polymorphism in the human P2X4 receptor is associated with increased pulse pressure. Hypertension 58:1086–1092. https://doi.org/10.1161/HYPERTENSIONAHA.111.176180
Center for Disease Control and Prevention. Chronic kidney disease surveillance system - United States. http://www.cdc.gov/ckd
Chen TK, Knicely DH, Grams ME (2019) Chronic kidney disease diagnosis and management: a review. JAMA 322:1294–1304. https://doi.org/10.1001/jama.2019.14745
Granata S, Masola V, Zoratti E, Scupoli MT, Baruzzi A, Messa M, Sallustio F, Gesualdo L, Lupo A, Zaza G (2015) NLRP3 inflammasome activation in dialyzed chronic kidney disease patients. PLoS ONE 10:e0122272. https://doi.org/10.1371/journal.pone.0122272
Solini A, Menini S, Rossi C, Ricci C, Santini E, Blasetti Fantauzzi C, Iacobini C, Pugliese G (2013) The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation. J Pathol 231:342–353. https://doi.org/10.1002/path.4237
Lajdova I, Oksa A, Horvathova M, Spustova V (2017) Expression of purinergic P2X7 receptors in subpopulations of peripheral blood mononuclear cells in early-stage of chronic kidney disease. J Physiol Pharmacol : Off J Polish Physiol Soc 68:779–785
Lajdova I, Oksa A, Chorvat D Jr, Topor P, Spustova V (2012) Purinergic P2X7 receptors participate in disturbed intracellular calcium homeostasis in peripheral blood mononuclear cells of patients with chronic kidney disease. Kidney Blood Press Res 35:48–57. https://doi.org/10.1159/000330349
Potthoff SA, Stegbauer J, Becker J, Wagenhaeuser PJ, Duvnjak B, Rump LC, Vonend O (2013) P2Y2 receptor deficiency aggravates chronic kidney disease progression. Front Physiol 4:234. https://doi.org/10.3389/fphys.2013.00234
Rabadi M, Kim M, Li H, Han SJ, Choi Y, D’Agati V, Lee HT (2018) ATP induces PAD4 in renal proximal tubule cells via P2X7 receptor activation to exacerbate ischemic AKI. Am J Physiol Renal Physiol 314:F293–F305. https://doi.org/10.1152/ajprenal.00364.2017
Hsu RK, Hsu C-Y (2016) The role of acute kidney injury in chronic kidney disease. Semin Nephrol 36:283–292. https://doi.org/10.1016/j.semnephrol.2016.05.005
Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS (2008) The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74:101–107. https://doi.org/10.1038/ki.2008.107
Bellomo R, Kellum JA, Ronco C (2012) Acute kidney injury. Lancet 380:756–766. https://doi.org/10.1016/S0140-6736(11)61454-2
Han SJ, Lovaszi M, Kim M, D’Agati V, Haskó G, Lee HT (2020) P2X4 receptor exacerbates ischemic AKI and induces renal proximal tubular NLRP3 inflammasome signaling. Faseb J 34:5465–5482. https://doi.org/10.1096/fj.201903287R
Yan Y, Bai J, Zhou X, Tang J, Jiang C, Tolbert E, Bayliss G, Gong R, Zhao TC, Zhuang S (2015) P2X7 receptor inhibition protects against ischemic acute kidney injury in mice. Am J Physiol Cell Physiol 308:C463-472. https://doi.org/10.1152/ajpcell.00245.2014
Battistone MA, Mendelsohn AC, Spallanzani RG, Allegretti AS, Liberman RN, Sesma J, Kalim S, Wall SM, Bonventre JV, Lazarowski ER, Brown D, Breton S(2020) Pro-inflammatory P2Y14 receptor inhibition protects against ischemic acute kidney injury in mice. J Clin Investig. https://doi.org/10.1172/JCI134791
Polycystic kidney disease. U.S. National Library of Medicine (2020).
Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE (2018) Polycystic kidney disease. Nat Rev Dis Primers 4:50–50. https://doi.org/10.1038/s41572-018-0047-y
Turner CM, Ramesh B, Srai SK, Burnstock G, Unwin RJ (2004) Altered ATP-sensitive P2 receptor subtype expression in the Han:SPRD cy/+ rat, a model of autosomal dominant polycystic kidney disease. Cells Tissues Organs 178:168–179. https://doi.org/10.1159/000082247
Chang MY, Lu JK, Tian YC, Chen YC, Hung CC, Huang YH, Chen YH, Wu MS, Yang CW, Cheng YC (2011) Inhibition of the P2X7 receptor reduces cystogenesis in PKD. J Am Soc Nephrol 22:1696–1706. https://doi.org/10.1681/asn.2010070728
Hillman KA, Woolf AS, Johnson TM, Wade A, Unwin RJ, Winyard PJ (2004) The P2X7 ATP receptor modulates renal cyst development in vitro. Biochem Biophys Res Commun 322:434–439. https://doi.org/10.1016/j.bbrc.2004.07.148
Schwiebert EM, Wallace DP, Braunstein GM, King SR, Peti-Peterdi J, Hanaoka K, Guggino WB, Guay-Woodford LM, Bell PD, Sullivan LP, Grantham JJ, Taylor AL (2002) Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol-Renal Physiol 282:F763–F775. https://doi.org/10.1152/ajprenal.0337.2000
Wilson PD, Hovater JS, Casey CC, Fortenberry JA, Schwiebert EM (1999) ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J Am Soc Nephrol 10:218–229
Palygin O, Ilatovskaya DV, Levchenko V, Klemens CA, Dissanayake L, Williams AM, Pavlov TS, Staruschenko A (2018) Characterization of purinergic receptor expression in ARPKD cystic epithelia. Purinergic Signal 14:485–497. https://doi.org/10.1007/s11302-018-9632-5
Linehan WM, Ricketts CJ (2019) The cancer genome atlas of renal cell carcinoma: findings and clinical implications. Nat Rev Urol 16:539–552. https://doi.org/10.1038/s41585-019-0211-5
Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017
Maynard JP, Lee J-S, Sohn BH, Yu X, Lopez-Terrada D, Finegold MJ, Goss JA, Thevananther S (2015) P2X3 purinergic receptor overexpression is associated with poor recurrence-free survival in hepatocellular carcinoma patients. Oncotarget 6:41162–41179
Nylund G, Hultman L, Nordgren S, Delbro DS (2007) P2Y2- and P2Y4 purinergic receptors are over-expressed in human colon cancer. Auton Autacoid Pharmacol 27:79–84. https://doi.org/10.1111/j.1474-8673.2007.00389.x
Künzli BM, Berberat PO, Giese T, Csizmadia E, Kaczmarek E, Baker C, Halaceli I, Büchler MW, Friess H, Robson SC (2007) Upregulation of CD39/NTPDases and P2 receptors in human pancreatic disease. Am J Physiol - Gastrointest Liver Physiol 292:G223–G230
Liu Z, Liu Y, Xu L, An H, Chang Y, Yang Y, Zhang W, Xu J (2015) P2X7 receptor predicts postoperative cancer-specific survival of patients with clear-cell renal cell carcinoma. Cancer Sci 106:1224–1231. https://doi.org/10.1111/cas.12736
Gong D, Zhang J, Chen Y, Xu Y, Ma J, Hu G, Huang Y, Zheng J, Zhai W, Xue W (2019) The m(6)A-suppressed P2RX6 activation promotes renal cancer cells migration and invasion through ATP-induced Ca(2+) influx modulating ERK1/2 phosphorylation and MMP9 signaling pathway. J Exp Clin Cancer Res: CR 38:233. https://doi.org/10.1186/s13046-019-1223-y
Yusenko MV, Kuiper RP, Boethe T, Ljungberg B, van Kessel AG, Kovacs G (2009) High-resolution DNA copy number and gene expression analyses distinguish chromophobe renal cell carcinomas and renal oncocytomas. BMC Cancer 9:152. https://doi.org/10.1186/1471-2407-9-152
Cutcliffe C, Kersey D, Huang CC, Zeng Y, Walterhouse D, Perlman EJ (2005) Clear cell sarcoma of the kidney: up-regulation of neural markers with activation of the sonic hedgehog and Akt pathways. Clin Cancer Res 11:7986–7994. https://doi.org/10.1158/1078-0432.ccr-05-1354
Gumz ML, Zou H, Kreinest PA, Childs AC, Belmonte LS, LeGrand SN, Wu KJ, Luxon BA, Sinha M, Parker AS, Sun LZ, Ahlquist DA, Wood CG, Copland JA (2007) Secreted frizzled-related protein 1 loss contributes to tumor phenotype of clear cell renal cell carcinoma. Clin Cancer Res 13:4740–4749. https://doi.org/10.1158/1078-0432.ccr-07-0143
Beroukhim R, Brunet JP, Di Napoli A, Mertz KD, Seeley A, Pires MM, Linhart D, Worrell RA, Moch H, Rubin MA, Sellers WR, Meyerson M, Linehan WM, Kaelin WG Jr, Signoretti S (2009) Patterns of gene expression and copy-number alterations in von-hippel lindau disease-associated and sporadic clear cell carcinoma of the kidney. Cancer Res 69:4674–4681. https://doi.org/10.1158/0008-5472.can-09-0146
Díez-Villanueva A, Mallona I, Peinado MA (2015) Wanderer, an interactive viewer to explore DNA methylation and gene expression data in human cancer. Epigenetics Chromatin 8:22. https://doi.org/10.1186/s13072-015-0014-8
Ruggieri MR Sr (2006) Mechanisms of disease: role of purinergic signaling in the pathophysiology of bladder dysfunction. Nat Clin Pract Urol 3:206–215. https://doi.org/10.1038/ncpuro0456
Takezawa K, Kondo M, Nonomura N, Shimada S (2017) Urothelial ATP signaling: what is its role in bladder sensation? Neurourol Urodyn 36:966–972. https://doi.org/10.1002/nau.23099
Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G (2004) Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol 287:F1084–F1091. https://doi.org/10.1152/ajprenal.00118.2004
Silva I, Ferreirinha F, Magalhães-Cardoso MT, Silva-Ramos M, Correia-de-Sá P (2015) Activation of P2Y6 receptors facilitates nonneuronal adenosine triphosphate and acetylcholine release from urothelium with the lamina propria of men with bladder outlet obstruction. J Urol 194:1146–1154. https://doi.org/10.1016/j.juro.2015.05.080
Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA, Ferguson DR (2004) P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int 93:1344–1348. https://doi.org/10.1111/j.1464-410X.2004.04858.x
Liu H-T, Kuo H-C (2017) Expressions of urothelial functional proteins in idiopathic detrusor overactivity patients refractory to antimuscarinic therapy with different urodynamic characteristics. Neurourol Urodyn 36:1313–1319. https://doi.org/10.1002/nau.23138
Shabir S, Cross W, Kirkwood LA, Pearson JF, Appleby PA, Walker D, Eardley I, Southgate J (2013) Functional expression of purinergic P2 receptors and transient receptor potential channels by the human urothelium. Am J Physiol Renal Physiol 305:F396-406. https://doi.org/10.1152/ajprenal.00127.2013
Kira S, Yoshiyama M, Tsuchiya S, Shigetomi E, Miyamoto T, Nakagomi H, Shibata K, Mochizuki T, Takeda M, Koizumi S (2017) P2Y6-deficiency increases micturition frequency and attenuates sustained contractility of the urinary bladder in mice. Sci Rep 7:771. https://doi.org/10.1038/s41598-017-00824-2
Liu M, Xu Y-F, Feng Y, Yang F-Q, Luo J, Zhai W, Che J-P, Wang G-C, Zheng J-H (2013) Epigallocatechin gallate attenuates interstitial cystitis in human bladder urothelium cells by modulating purinergic receptors. J Surg Res 183:397–404. https://doi.org/10.1016/j.jss.2012.11.041
Vacca F, D’Ambrosi N, Nestola V, Amadio S, Giustizieri M, Cucchiaroni ML, Tozzi A, Velluz MC, Mercuri NB, Volonte C (2011) N-Glycans mutations rule oligomeric assembly and functional expression of P2X3 receptor for extracellular ATP. Glycobiology 21:634–643. https://doi.org/10.1093/glycob/cwq211
Irwin GM (2019) Urinary incontinence. Prim Care: Clin Off Practice 46:233–242. https://doi.org/10.1016/j.pop.2019.02.004
Carneiro I, Timoteo MA, Silva I, Vieira C, Baldaia C, Ferreirinha F, Silva-Ramos M, Correia-de-Sa P (2014) Activation of P2Y6 receptors increases the voiding frequency in anaesthetized rats by releasing ATP from the bladder urothelium. Br J Pharmacol 171:3404–3419. https://doi.org/10.1111/bph.12711
Moore KH, Ray FR, Barden JA (2001) Loss of purinergic P2X3 and P2X5 receptor innervation in human detrusor from adults with urge incontinence. J Neurosci 21:RC166–RC166. https://doi.org/10.1523/JNEUROSCI.21-18-j0002.2001
Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK, Schaer GN (2010) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J 21:5–26. https://doi.org/10.1007/s00192-009-0976-9
Firouzmand S, Ajori L, Towse J, Allameh F, Najafi S, Javed S, John B, Langley SEM, Fry CH, Young JS (2020) Investigating the associations of mucosal P2Y6 receptor expression and urinary ATP and ADP concentrations, with symptoms of overactive bladder. Neurourol Urodyn 39:926–934. https://doi.org/10.1002/nau.24322
Hao Y, Wang L, Chen H, Hill WG, Robson SC, Zeidel ML, Yu W (2019) Targetable purinergic receptors P2Y12 and A2b antagonistically regulate bladder function. JCI Insight 4(16):e122112. https://doi.org/10.1172/jci.insight.122112
Sterle I, Zupancic D, Romih R (2014) Correlation between urothelial differentiation and sensory proteins P2X3, P2X5, TRPV1, and TRPV4 in normal urothelium and papillary carcinoma of human bladder. Biomed Res Int 2014:805236. https://doi.org/10.1155/2014/805236
Ding M, Zhan H, Liao X, Li A, Zhong Y, Gao Q, Liu Y, Huang W, Cai Z (2018) Enhancer RNA - P2RY2e induced by estrogen promotes malignant behaviors of bladder cancer. Int J Biol Sci 14:1268–1276. https://doi.org/10.7150/ijbs.27151
Puppo V, Puppo G (2016) Comprehensive review of the anatomy and physiology of male ejaculation: premature ejaculation is not a disease. Clin Anat 29:111–119. https://doi.org/10.1002/ca.22655
Haynes JM, Ventura S (2005) Current models of human prostate contractility. Clin Exp Pharmacol Physiol 32:797–804. https://doi.org/10.1111/j.1440-1681.2005.04268.x
White CW, Short JL, Evans RJ, Ventura S (2015) Development of a P2X1-purinoceptor mediated contractile response in the aged mouse prostate gland through slowing down of ATP breakdown. Neurourol Urodyn 34:292–298. https://doi.org/10.1002/nau.22519
Rodríguez D, Olumi AF (2012) Management of spermatic cord tumors: a rare urologic malignancy. Ther Adv Urol 4:325–334. https://doi.org/10.1177/1756287212447839
Ramamurthy R, Periasamy S, Mettupalayam V (2011) Primary malignancy of seminal vesicle: a rare entity. Indian J Urol 27:137–139. https://doi.org/10.4103/0970-1591.78417
Yeung C-H, Wang K, Cooper TG (2012) Why are epididymal tumours so rare? Asian J Androl 14:465–475. https://doi.org/10.1038/aja.2012.20
Sutcliffe S, Zenilman JM, Ghanem KG, Jadack RA, Sokoll LJ, Elliott DJ, Nelson WG, De Marzo AM, Cole SR, Isaacs WB, Platz EA (2006) Sexually transmitted infections and prostatic inflammation/cell damage as measured by serum prostate specific antigen concentration. J Urol 175:1937–1942. https://doi.org/10.1016/s0022-5347(05)00892-x
Shrestha E, White JR, Yu SH, Kulac I, Ertunc O, De Marzo AM, Yegnasubramanian S, Mangold LA, Partin AW, Sfanos KS (2018) Profiling the urinary microbiome in men with positive versus negative biopsies for prostate cancer. J Urol 199:161–171. https://doi.org/10.1016/j.juro.2017.08.001
Slater M, Barden JA, Murphy CR (2000) The purinergic calcium channels P2X1,2,5,7 are down-regulated while P2X3,4,6 are up-regulated during apoptosis in the ageing rat prostate. Histochem J 32:571–580. https://doi.org/10.1023/a:1004110529753
Krieger JN, Lee SWH, Jeon J, Cheah PY, Liong ML, Riley DE (2008) Epidemiology of prostatitis. Int J Antimicrob Agents 31:85–90. https://doi.org/10.1016/j.ijantimicag.2007.08.028
Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP (2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407:1011–1015. https://doi.org/10.1038/35039519
Zhang H, Liu L, Lu G, Chen Z, Fang Q, Yang Z, Li L, Li W, Song B, Zhou Z (2011) Chemical irritation of the prostate sensitizes P(2)X(3) receptor-mediated responses in rat dorsal root ganglion neurons. Neurourol Urodyn 30:612–618. https://doi.org/10.1002/nau.21060
Schwartz ES, Xie A, La JH, Gebhart GF (2015) Nociceptive and inflammatory mediator upregulation in a mouse model of chronic prostatitis. Pain 156:1537–1544. https://doi.org/10.1097/j.pain.0000000000000201
Zhang H, Liu L, Yang Z, Pan J, Chen Z, Fang Q, Li W, Li L, Lu G, Zhou Z (2013) P2X7 receptor mediates activation of microglial cells in prostate of chemically irritated rats. Int Braz J Urol 39:276–285. https://doi.org/10.1590/S1677-5538.IBJU.2013.02.17
Cheng Y, Mansfield KJ, Allen W, Chess-Williams R, Burcher E, Moore KH (2014) ATP during early bladder stretch is important for urgency in detrusor overactivity patients. Biomed Res Int 2014:204604. https://doi.org/10.1155/2014/204604
Abbasian B, Shair A, O'Gorman DB, Pena-Diaz AM, Brennan L, Engelbrecht K, Koenig DW, Reid G, Burton JP (2019) Potential role of extracellular ATP released by bacteria in bladder infection and contractility. mSphere 4:e00439–e00419. https://doi.org/10.1128/mSphere.00439-19
de Rivero Vaccari JP, Bastien D, Yurcisin G, Pineau I, Dietrich WD, De Koninck Y, Keane RW, Lacroix S (2012) P2X4 receptors influence inflammasome activation after spinal cord injury. J Neurosci: Off J Soc Neurosci 32:3058–3066. https://doi.org/10.1523/jneurosci.4930-11.2012
Csóka B, Németh ZH, Szabó I, Davies DL, Varga ZV, Pálóczi J, Falzoni S, Di Virgilio F, Muramatsu R, Yamashita T, Pacher P, Haskó G (2018) Macrophage P2X4 receptors augment bacterial killing and protect against sepsis. JCI Insight 3. https://doi.org/10.1172/jci.insight.99431
Wei Q, Costanzi S, Liu QZ, Gao ZG, Jacobson KA (2011) Activation of the P2Y1 receptor induces apoptosis and inhibits proliferation of prostate cancer cells. Biochem Pharmacol 82:418–425. https://doi.org/10.1016/j.bcp.2011.05.013
Li WH, Qiu Y, Zhang HQ, Liu Y, You JF, Tian XX, Fang WG (2013) P2Y2 receptor promotes cell invasion and metastasis in prostate cancer cells. Br J Cancer 109:1666–1675. https://doi.org/10.1038/bjc.2013.484
Lertsuwan K et al (2017) Purinergic receptor expression and cellular responses to purinergic agonists in human prostate cancer cells. Anticancer Res 37:529–537. https://doi.org/10.21873/anticanres.11345
Maynard JP, Lu J, Vidal I, Hicks J, Mummert L, Ali T, Kempski R, Carter AM, Sosa RY, Peiffer LB, Joshu CE, Lotan TL, De Marzo AM, Sfanos KS (2022) P2X4 purinergic receptors offer a therapeutic target for aggressive prostate cancer. J Pathol 256(2):149–63 https://doi.org/10.1002/path.5815
Shabbir M, Ryten M, Thompson C, Mikhailidis D, Burnstock G (2008) Characterization of calcium-independent purinergic receptor-mediated apoptosis in hormone-refractory prostate cancer. BJU Int 101:352–359. https://doi.org/10.1111/j.1464-410X.2007.07293.x
Nandigama R, Padmasekar M, Wartenberg M, Sauer H (2006) Feed forward cycle of hypotonic stress-induced ATP release, purinergic receptor activation, and growth stimulation of prostate cancer cells. J Biol Chem 281:5686–5693. https://doi.org/10.1074/jbc.M510452200
Chen L, He H-y, Li H-m, Zheng J, Heng W-j, You J-f, Fang W-g (2004) ERK1/2 and p38 pathways are required for P2Y receptor-mediated prostate cancer invasion. Cancer Lett 215:239–247. https://doi.org/10.1016/j.canlet.2004.05.023
Zhang Y, Gong L-h, Zhang H-q, Du Q, You J-f, Tian X-x, Fang W-g (2010) Extracellular ATP enhances in vitro invasion of prostate cancer cells by activating Rho GTPase and upregulating MMPs expression. Cancer Lett 293:189–197. https://doi.org/10.1016/j.canlet.2010.01.010
Slater M, Danieletto S, Barden JA (2005) Expression of the apoptotic calcium channel P2X7 in the glandular epithelium. J Mol Histol 36:159–165. https://doi.org/10.1007/s10735-004-6166-7
Gilbert SM, Oliphant CJ, Hassan S, Peille AL, Bronsert P, Falzoni S, Di Virgilio F, McNulty S, Lara R (2019) ATP in the tumour microenvironment drives expression of nfP2X7, a key mediator of cancer cell survival. Oncogene 38:194–208. https://doi.org/10.1038/s41388-018-0426-6
Ghalali A, Wiklund F, Zheng H, Stenius U, Högberg J (2014) Atorvastatin prevents ATP-driven invasiveness via P2X7 and EHBP1 signaling in PTEN-expressing prostate cancer cells. Carcinogenesis 35:1547–1555. https://doi.org/10.1093/carcin/bgu019
Solini A, Simeon V, Derosa L, Orlandi P, Rossi C, Fontana A, Galli L, Di Desidero T, Fioravanti A, Lucchesi S, Coltelli L, Ginocchi L, Allegrini G, Danesi R, Falcone A, Bocci G (2015) Genetic interaction of P2X7 receptor and VEGFR-2 polymorphisms identifies a favorable prognostic profile in prostate cancer patients. Oncotarget 6:28743–28754. https://doi.org/10.18632/oncotarget.4926
He J, Zhou Y, Arredondo Carrera HM, Sprules A, Neagu R, Zarkesh SA, Eaton C, Luo J, Gartland A, Wang N (2020) Inhibiting the P2X4 receptor suppresses prostate cancer growth in vitro and in vivo, suggesting a potential clinical target. Cells 9(11). https://doi.org/10.3390/cells9112511
Haney NM, Faisal FA, Lu J, Guedes LB, Reuter VE, Scher HI, Eastham JA, Marchionni L, Joshu C, Gopalan A, Lotan TL (2020) PTEN loss with ERG negative status is associated with lethal disease after radical prostatectomy. J Urol 203:344–350. https://doi.org/10.1097/ju.0000000000000533
Ghalali A, Ye Z-W, Högberg J, Stenius U (2020) PTEN and PHLPP crosstalk in cancer cells and in TGFβ-activated stem cells. Biomed Pharmacother 127:110112. https://doi.org/10.1016/j.biopha.2020.110112
Ahearn TU, Pettersson A, Ebot EM, Gerke T, Graff RE, Morais CL, Hicks JL, Wilson KM, Rider JR, Sesso HD, Fiorentino M, Flavin R, Finn S, Giovannucci EL, Loda M, Stampfer MJ, De Marzo AM, Mucci LA, Lotan TL (2016) A Prospective Investigation of PTEN Loss and ERG Expression in Lethal Prostate Cancer. J Natl Cancer Inst 108(2). https://doi.org/10.1093/jnci/djv346
Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science (New York, N.Y.) 310:644–648. https://doi.org/10.1126/science.1117679
Tian TV, Tomavo N, Huot L, Flourens A, Bonnelye E, Flajollet S, Hot D, Leroy X, de Launoit Y, Duterque-Coquillaud M (2014) Identification of novel TMPRSS2:ERG mechanisms in prostate cancer metastasis: involvement of MMP9 and PLXNA2. Oncogene 33:2204–2214. https://doi.org/10.1038/onc.2013.176
Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ (2000) Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 31:578–583. https://doi.org/10.1053/hp.2000.6698
Falk S, Uldall M, Heegaard A-M (2012) The role of purinergic receptors in cancer-induced bone pain. J Osteoporos 758181–758181:2012. https://doi.org/10.1155/2012/758181
Yan Y, Liang Y, Ding T, Chu H (2019) PI3K/Akt signaling pathway may be involved in MCP-1-induced P2X4R expression in cultured microglia and cancer-induced bone pain rats. Neurosci Lett 701:100–105. https://doi.org/10.1016/j.neulet.2019.02.024
Jin X-H, Wang L-N, Zuo J-L, Yang J-P, Liu S-L (2014) P2X4 receptor in the dorsal horn partially contributes to brain-derived neurotrophic factor oversecretion and toll-like receptor-4 receptor activation associated with bone cancer pain. J Neurosci Res 92:1690–1702. https://doi.org/10.1002/jnr.23443
Calvert RC, Khan MA, Thompson CS, Mikhailidis DP, Burnstock G (2008) A functional study of purinergic signalling in the normal and pathological rabbit corpus cavernosum. BJU Int 101:1043–1047. https://doi.org/10.1111/j.1464-410X.2007.07385.x
Gur S, Hellstrom WJ (2009) Activation of P2Y1 and P2Y2 nucleotide receptors by adenosine 5’-triphosphate analogues augmented nerve-mediated relaxation of human corpus cavernosum. Can Urol Assoc J = J l’Assoc Urol Can 3:314–318. https://doi.org/10.5489/cuaj.1127
Lau DH, Metcalfe MJ, Mumtaz FH, Mikhailidis DP, Thompson CS (2009) Purinergic modulation of human corpus cavernosum relaxation. Int J Androl 32:149–155. https://doi.org/10.1111/j.1365-2605.2007.00828.x
Odom MR, Pak ES, Brown DA, Hannan JL (2017) Enhanced electrical field stimulated nitrergic and purinergic vasoreactivity in distal vs proximal internal pudendal arteries. J Sex Med 14:1285–1296. https://doi.org/10.1016/j.jsxm.2017.09.013
Gur S, Kadowitz PJ, Abdel-Mageed AB, Kendirci M, Sikka SC, Burnstock G, Hellstrom WJG (2009) Management of erectile function by penile purinergic p2 receptors in the diabetic rat. J Urol 181:2375–2382. https://doi.org/10.1016/j.juro.2009.01.002
Gur S, Sikka SC, Knight GE, Burnstock G, Hellstrom WJG (2010) Purinergic contraction of the rat vas deferens in L-NAME-induced hypertension: effect of sildenafil. Asian J Androl 12:415–421. https://doi.org/10.1038/aja.2009.70
Shalev M, Staerman F, Allain H, Lobel B, Saiag B (1999) Stimulation of P2y purinoceptors induces, via nitric oxide production, endothelium-dependent relaxation of human isolated corpus cavernosum. J Urol 161:955–959
Kilicarslan H, Yildirim S, Bagcivan I, Ayan S, Sarac B, Sarioglu Y (2002) Effect of chronic renal failure on the purinergic responses of corpus cavernosal smooth muscle in rabbits. BJU Int 90:596–600. https://doi.org/10.1046/j.1464-410x.2002.02979.x
Yildirim MK, Bagcivan I, Sarac B, Kilicarslan H, Yildirim S, Kaya T (2008) Effect of hypothyroidism on the purinergic responses of corpus cavernosal smooth muscle in rabbits. Int Urol Nephrol 40:691–699. https://doi.org/10.1007/s11255-008-9332-0
Faria M, Magalhaes-Cardoso T, Lafuente-de-Carvalho JM, Correia-de Sa P (2008) Decreased ecto-NTPDase1/CD39 activity leads to desensitization of P2 purinoceptors regulating tonus of corpora cavernosa in impotent men with endothelial dysfunction. Nucleosides, Nucleotides and Nucleic Acids 27:761–768. https://doi.org/10.1080/15257770802145744
Liu P, Jiang J, Xia J, Jiang R (2018) Effect of low androgen status on the expression of P2Y receptors in the corpus cavernosum of rats. Urology 116(229):e221-229.e226. https://doi.org/10.1016/j.urology.2018.03.018
Li CL, Yang XL, Wang JJ, Du GH, Yang WM, Zhang HP (2015) Effects of intracavernous injection of P2X3 and NK1 receptor antagonists on erectile dysfunction induced by spinal cord transection in rats. Andrologia 47:25–29. https://doi.org/10.1111/and.12217
Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, Evans RJ (2000) Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature 403:86–89. https://doi.org/10.1038/47495
White CW, Choong YT, Short JL, Exintaris B, Malone DT, Allen AM, Evans RJ, Ventura S (2013) Male contraception via simultaneous knockout of alpha1A-adrenoceptors and P2X1-purinoceptors in mice. Proc Natl Acad Sci USA 110:20825–20830. https://doi.org/10.1073/pnas.1318624110
Walenta L, Fleck D, Frohlich T, von Eysmondt H, Arnold GJ, Spehr J, Schwarzer JU, Kohn FM, Spehr M, Mayerhofer A (2018) ATP-mediated events in peritubular cells contribute to sterile testicular inflammation. Sci Rep 8:1431. https://doi.org/10.1038/s41598-018-19624-3
Fleck D, Kenzler L, Mundt N, Strauch M, Uesaka N, Moosmann R, Bruentgens F, Missel A, Mayerhofer A, Merhof D, Spehr J, Spehr M (2021) ATP activation of peritubular cells drives testicular sperm transport. Elife 10:e62885. https://doi.org/10.7554/eLife.62885
Giuliani AL, Sarti AC and Di Virgilio F (2021) Ectonucleotidases in acute and chronic inflammation. Front Pharmacol 11:619458. https://doi.org/10.3389/fphar.2020.619458
Zylka MJ (2011) Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol Med 17:188–196. https://doi.org/10.1016/j.molmed.2010.12.006
Haas CB, Lovászi M, Braganhol E, Pacher P, Haskó G (2021) (2021) Ectonucleotidases in inflammation immunity and cancer. J Immunol Baltimore (Baltimore, Md. : 1950) 206:1983–1990
Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A (2013) ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene 32:1743–1751. https://doi.org/10.1038/onc.2012.269
Dwyer KM, Kishore BK, Robson SC (2020) Conversion of extracellular ATP into adenosine: a master switch in renal health and disease. Nat Rev Nephrol 16:509–524. https://doi.org/10.1038/s41581-020-0304-7
Yu W, Robson SC, Hill WG (2011) Expression and distribution of ectonucleotidases in mouse urinary bladder. PLoS ONE 6:e18704. https://doi.org/10.1371/journal.pone.0018704
Yu W, Sun X, Robson SC, Hill WG (2014) ADP-induced bladder contractility is mediated by P2Y12 receptor and temporally regulated by ectonucleotidases and adenosine signaling. FASEB J 28:5288–5298. https://doi.org/10.1096/fj.14-255885
Kauffenstein G, Pelletier J, Lavoie EG, Kukulski F, Martín-Satué M, Dufresne SS, Frenette J, Ribas Fürstenau C, Sereda MJ, Toutain B, Henrion D, Sullivan R, Vial C, Sévigny J (2014) Nucleoside triphosphate diphosphohydrolase-1 ectonucleotidase is required for normal vas deferens contraction and male fertility through maintaining P2X1 receptor function*. J Biol Chem 289:28629–28639. https://doi.org/10.1074/jbc.M114.604082
Ferreira JM, Matheus LHG, Almeida RVSd, Melo PAdS, Leite KRM, Murta CB, Claro JFdA, Camacho CP, Pontes-Júnior J, Dellê H (2021) High CD39 expression is associated with the non-muscle-invasive phenotype of human bladder cancer. Oncotarget 12(16):1580–1586. https://doi.org/10.18632/oncotarget.28029
Gardani CFF, Cappellari AR, de Souza JB, da Silva BT, Engroff P, Moritz CEJ, Scholl JN, Battastini AMO, Figueiró F, Morrone FB (2019) Hydrolysis of ATP, ADP, and AMP is increased in blood plasma of prostate cancer patients. Purinergic Signalling 15:95–105. https://doi.org/10.1007/s11302-018-9642-3
Battisti V, Maders LDK, Bagatini MD, Battisti IE, Bellé LP, Santos KF, Maldonado PA, Thomé GR, Schetinger MRC, Morsch VM (2013) Ectonucleotide pyrophosphatase/phosphodiesterase (E-NPP) and adenosine deaminase (ADA) activities in prostate cancer patients: influence of Gleason score, treatment and bone metastasis. Biomed Pharmacother 67:203–208. https://doi.org/10.1016/j.biopha.2012.12.004
Plavix/Clopidogrel bisulfate Drug Approval Package (1997) U. S. Food & Drug Administration. www.accessdata.fda.gov/drugsatfda_docs/nda/97/020839_plavix_toc.cfm. Accessed 24 Nov 2020
The safety and efficacy of gefapixant (AF-219/MK-7264) in female subjects with interstitial cystitis bladder pain syndrome. ClinicalTrials.gov identifier: NCT01569438. Updated 17 August 2020. https://ClinicalTrials.gov/show/NCT01569438
A four-week multicenter study evaluating the safety and efficacy of gefapixant (AF-219/MK-7264) in subjects with osteoarthritis of the knee. ClinicalTrials.gov identifier: NCT01554579. Updated 27 June 2019.https://ClinicalTrials.gov/show/NCT01554579
Efficacy and safety of gefapixant (MK-7264) in women with chronic cough and stress Urinary Incontinence (MK-7264-042). ClinicalTrials.gov identifier: NCT04193176. Updated 26 April 2022. https://ClinicalTrials.gov/show/NCT04193176
A Randomised, Placebo-controlled, Double-blind Trial of the Antidepressant Efficacy of a Novel CNS-penetrant P2X7 Receptor Antagonist, JNJ-54175446, in People With Major Depressive Disorder, an Incomplete Response to Monoaminergic Antidepressant Drugs, and a Biomarker Profile Predictive of Active P2X7 Signalling. ClinicalTrials.gov identifier: NCT04116606. Updated 11 May 2022.https://ClinicalTrials.gov/show/NCT04116606
A PHASE 2A, Randomized, double-blind, placebo-controlled, parallel-group study of CE-224,535, an antagonist of the P2X7 receptor, in the treatment of the signs and symptoms of rheumatoid arthritis in subjects who are inadequately controlled on Methotrexate. ClinicalTrials.gov identifier: NCT00628095. Updated April 2022. https://ClinicalTrials.gov/show/NCT00628095
A Randomised, Double-Blind (With Open Comparator Etanercept Limb), Placebo-Controlled, Phase IIb, Multicentre Study to Evaluate the Efficacy of 4 Doses of AZD9056 Administered for 6 Months on the Signs and Symptoms of Rheumatoid Arthritis in Patients With Active Disease Receiving Background Methotrexate or Sulphasalazine. ClinicalTrials.gov identifier: NCT00520572. Updated February 2013. https://ClinicalTrials.gov/show/NCT00520572
A First-Time-in-Human Randomised, Single Blind Placebo-Controlled Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics, of Single Escalating Doses of GSK1482160, in Male and Female Healthy Subjects, and to Make a Preliminary Assessment of the Effect of Food. ClinicalTrials.gov identifier: NCT00849134. Updated 7 July 2017. https://ClinicalTrials.gov/show/NCT00849134
Ali Z, Laurijssens B, Ostenfeld T, McHugh S, Stylianou A, Scott-Stevens P, Hosking L, Dewit O, Richardson JC, Chen C (2013) Pharmacokinetic and pharmacodynamic profiling of a P2X7 receptor allosteric modulator GSK1482160 in healthy human subjects. Br J Clin Pharmacol 75:197–207. https://doi.org/10.1111/j.1365-2125.2012.04320.x
A Double-Blind, Placebo-Controlled, Randomized Single and Multiple Ascending Dose Study to Investigate the Safety, Tolerability, and Pharmacokinetics of JNJ-55308942 in Healthy Male and Female Subjects. ClinicalTrials.gov identifier: NCT03151486. Updated 19 April 2018 https://ClinicalTrials.gov/show/NCT03151486
An Open-Label Phase 1 Investigation of the Safety and Tolerability of BSCT (Anti-nf-P2X7) 10% Ointment When Applied Twice Daily for 28 Days in Male and Female Patients With Basal Cell Carcinoma. ClinicalTrials.gov identifier: NCT002587819. Updated 4 April 2016. https://ClinicalTrials.gov/show/NCT02587819
An Open-label PET Study to Investigate P2X7 Receptor Occupancy by JNJ-55308942 Using [18F]-JNJ-64413739. ClinicalTrials.gov identifier: NCT03437590. Updated 23 January 2019 https://ClinicalTrials.gov/show/NCT03437590)
Gilbert SM, Gidley Baird A, Glazer S, Barden JA, Glazer A, Teh LC, King J (2017) A phase I clinical trial demonstrates that nfP2X(7) -targeted antibodies provide a novel, safe and tolerable topical therapy for basal cell carcinoma. Br J Dermatol 177:117–124. https://doi.org/10.1111/bjd.15364
A Multi-Center, Parallel Group, Double-Masked, Randomized, Placebo-Controlled Study of Multiple Ocular Instillations of Diquafosol Tetrasodium Ophthalmic Solution, 2% in Subjects With Dry Eye Disease. ClinicalTrials.gov identifier: NCT00403975. Updated 22 February 2016. https://ClinicalTrials.gov/show/NCT00403975
A Multi-Center, Parallel-Group, Double-Masked, Randomized, Placebo-Controlled Study of the Effects of Diquafosol Tetrasodium Ophthalmic Solution, 2% and Placebo in Subjects With Dry Eye Disease. ClinicalTrials.gov identifier: NCT02116010. Updated 9 January 2015.https://ClinicalTrials.gov/show/NCT00600288)
A Multi-Center, Double-Masked, Randomized, Placebo-Controlled, Dose-Ranging Study of Multiple Ocular Instillations of INS365 Ophthalmic Solution vs. Placebo in Subjects With Dry Eye Disease. ClinicalTrials.gov identifier: NCT00404131. Updated 29 November 2016https://ClinicalTrials.gov/show/NCT00404131)
SDouble-Masked, Randomized, Placebo-Controlled Study of Efficacy Parameter Following Administration of INS365 Ophthalmic Solution or Placebo in a Controlled Adverse Environment (CAE) Chamber in Subjects With Non-Sjogren's Associated Dry Eye. ClinicalTrials.gov identifier: NCT00037661. Updated 2 October 2015. (https://ClinicalTrials.gov/show/NCT00037661)
Mun Y, Kwon J-W, Oh JY (2018) Therapeutic effects of 3% diquafosol ophthalmic solution in patients with short tear film break-up time-type dry eye disease. BMC Ophthalmol 18:237–237. https://doi.org/10.1186/s12886-018-0910-3
Park DH, Chung JK, Seo DR, Lee SJ (2016) Clinical effects and safety of 3% diquafosol ophthalmic solution for patients with dry eye after cataract surgery: a randomized controlled trial. Am J Ophthalmol 163:122-131.e122. https://doi.org/10.1016/j.ajo.2015.12.002
A Phase 3, International, Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Study of Denufosol Tetrasodium Inhalation Solution in Patients With Cystic Fibrosis Lung Disease and FEV1 Greater Than or Equal to 75% Predicted But Less Than or Equal to 110% Predicted. ClinicalTrials.gov identifier: NCT00625612. Updated 1 November 2015 https://ClinicalTrials.gov/show/NCT00625612)
Ratjen F, Durham T, Navratil T, Schaberg A, Accurso FJ, Wainwright C, Barnes M, Moss RB (2012) Long term effects of denufosol tetrasodium in patients with cystic fibrosis. J Cyst Fibros 11:539–549. https://doi.org/10.1016/j.jcf.2012.05.003
de Baaij JH, Kompatscher A, Viering DH, Bos C, Bindels RJ, Hoenderop JG (2016) P2X6 knockout mice exhibit normal electrolyte homeostasis. PLoS ONE 11:e0156803. https://doi.org/10.1371/journal.pone.0156803
Vial C, Evans RJ (2001) Smooth muscle does not have a common P2X receptor phenotype: expression, ontogeny and function of P2x1 receptors in mouse ileum, bladder and reproductive systems. Auton Neurosci : Basic Clin 92:56–64. https://doi.org/10.1016/s1566-0702(01)00319-8
Banks FC, Calvert RC, Burnstock G (2010) Changing P2X receptor localization on maturing sperm in the epididymides of mice, hamsters, rats, and humans: a preliminary study. Fertil Steril 93:1415–1420. https://doi.org/10.1016/j.fertnstert.2009.02.061
Yu W, Hill WG, Robson SC, Zeidel ML (2018) Role of P2X4 receptor in mouse voiding function. Sci Rep 8:1838. https://doi.org/10.1038/s41598-018-20216-4
Suadicani SO, Urban-Maldonado M, Tar MT, Melman A, Spray DC (2009) Effects of ageing and streptozotocin-induced diabetes on connexin43 and P2 purinoceptor expression in the rat corpora cavernosa and urinary bladder. BJU Int 103:1686–1693. https://doi.org/10.1111/j.1464-410X.2008.08337.x
Wang ECY, Lee J-M, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G (2005) ATP and purinergic receptor–dependent membrane traffic in bladder umbrella cells. J Clin Investig 115:2412–2422. https://doi.org/10.1172/JCI24086
Guan NN, Sharma N, Hallén-Grufman K, Jager EWH, Svennersten K (2018) The role of ATP signalling in response to mechanical stimulation studied in T24 cells using new microphysiological tools. J Cell Mol Med 22:2319–2328. https://doi.org/10.1111/jcmm.13520
Shabbir M, Ryten M, Thompson C, Mikhailidis D, Burnstock G (2008) Purinergic receptor-mediated effects of ATP in high-grade bladder cancer. BJU Int 101:106–112. https://doi.org/10.1111/j.1464-410X.2007.07286.x
Acknowledgements
The results presented in Fig. 3 are based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga.
Funding
We would like to acknowledge research funding from the Department of Defense PCRP award W81XWH-17–1-0286 (K.S.S.) and award W81XWH-17–1-0292 (J.P.M), and from the Prostate Cancer Foundation Challenge Award 19CHAS03 (J.P.M. and K.S.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• P2 purinergic receptors are involved in the normal function of the kidney, bladder, and prostate and are also important for penile erection and fertility.
• P2 purinergic receptor dysregulation is reported in multiple urologic diseases and facilitates related inflammation and pain.
• Select P2 purinergic receptors are overexpressed in urologic cancers and are proposed therapeutic targets.
• Multiple P2 purinergic receptor antagonists are in clinical trials for treatment of inflammation-related disorders and pain causing optimism for novel therapeutic management of urologic diseases.
Rights and permissions
About this article
Cite this article
Maynard, J.P., Sfanos, K.S. P2 purinergic receptor dysregulation in urologic disease. Purinergic Signalling 18, 267–287 (2022). https://doi.org/10.1007/s11302-022-09875-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-022-09875-1