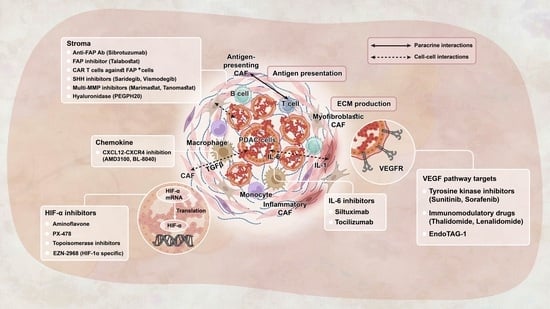
The Next Frontier in Pancreatic Cancer: Targeting the Tumor Immune Milieu and Molecular Pathways
Abstract
:Simple Summary
Abstract
1. Introduction
2. Immunotherapy
2.1. Immune Checkpoint Inhibitors (ICIs) and the Tumor Microenvironment (TME)
2.2. Vaccine Therapies
2.3. Cellular Therapies
2.4. Other Immunotherapy Approaches
3. Targeted Therapies
3.1. The DNA Damage Repair (DDR) Pathway
3.2. Targeting NTRK
3.3. Targeting KRAS
3.4. Targeting Downstream Effectors of KRAS
3.5. Targeting TGFβ Signaling
4. Metabolic Pathways
4.1. Targeting Asparagine
4.2. Targeting Glutamine
4.3. Targeting Adenosine Generating Enzyme
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 4 May 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Loveday, B.P.; Lipton, L.; Thomson, B.N. Pancreatic cancer: An update on diagnosis and management. Aust. J. Gen. Pract. 2019, 48, 826–831. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Amanam, I.; Chung, V. Current and future therapies for advanced pancreatic cancer. J. Surg. Oncol. 2017, 116, 25–34. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [Green Version]
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 2.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 22 February 2022).
- Luo, D.; Kuang, F.; Du, J.; Zhou, M.; Peng, F.; Gan, Y.; Fang, C.; Yang, X.; Li, B.; Su, S. Characterization of the Immune Cell Infiltration Profile in Pancreatic Carcinoma to Aid in Immunotherapy. Front. Oncol. 2021, 11, 1614. [Google Scholar] [CrossRef]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Ahmad-Nielsen, S.A.; Bruun Nielsen, M.F.; Mortensen, M.B.; Detlefsen, S. Frequency of mismatch repair deficiency in pancreatic ductal adenocarcinoma. Pathol. Res. Pract. 2020, 216, 152985. [Google Scholar] [CrossRef]
- Schizas, D.; Charalampakis, N.; Kole, C.; Kolea, C.; Economopoulou, P.; Koustas, E.; Gkotsis, E.; Ziogas, D.; Psyrri, A.; Karamouzis, M.V.; et al. Immunotherapy for pancreatic cancer: A 2020 update. Cancer Treat. Rev. 2020, 86, 102016. [Google Scholar] [CrossRef]
- Yoon, J.H.; Jung, Y.-J.; Moon, S.-H. Immunotherapy for pancreatic cancer. World J. Clin. Cases 2021, 9, 2969–2982. [Google Scholar] [CrossRef]
- Renouf, D.J.; Knox, J.J.; Kavan, P.; Jonker, D.; Welch, S.; Couture, F.; Lemay, F.; Tehfe, M.; Harb, M.; Aucoin, N.; et al. LBA65 The Canadian Cancer Trials Group PA.7 trial: Results of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) vs GEM, nab-P, durvalumab (D) and tremelimumab (T) as first line therapy in metastatic pancreatic ductal adenocarcinoma (mPDAC). Ann. Oncol. 2020, 31, S1195. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, Y.; Huang, D.; Guo, C.; Zhang, Q.; Li, X.; Zhang, X.; Gao, S.; Que, R.; Shen, Y.; et al. Randomized phase III study of sintilimab in combination with modified folfrinox versus folfrinox alone in patients with metastatic and recurrent pancreatic cancer in China: The CISPD3 trial. J. Clin. Oncol. 2022, 40, 560. [Google Scholar] [CrossRef]
- Melisi, D.; Oh, D.-Y.; Hollebecque, A.; Calvo, E.; Varghese, A.; Borazanci, E.; Macarulla, T.; Merz, V.; Zecchetto, C.; Zhao, Y.; et al. Safety and activity of the TGFβ receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J. Immunother. Cancer 2021, 9, e002068. [Google Scholar] [CrossRef]
- Parikh, A.R.; Szabolcs, A.; Allen, J.N.; Clark, J.W.; Wo, J.Y.; Raabe, M.; Thel, H.; Hoyos, D.; Mehta, A.; Arshad, S.; et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat. Cancer 2021, 2, 1124–1135. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G.J.; Waypa, J.; Blaydorn, L.; Coats, J.; McGahey, K.; Sangal, A.; Niu, J.; Lynch, C.A.; Farley, J.H.; Khemka, V. A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br. J. Cancer 2017, 117, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G.J.; Blaydorn, L.; Beck, J.; Bornemann-Kolatzki, K.; Urnovitz, H.; Schütz, E.; Khemka, V. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Investig. New Drugs 2018, 36, 96–102. [Google Scholar] [CrossRef]
- Doi, T.; Muro, K.; Ishii, H.; Kato, T.; Tsushima, T.; Takenoyama, M.; Oizumi, S.; Gemmoto, K.; Suna, H.; Enokitani, K.; et al. A phase 1 study of the anti-CC chemokine receptor 4 antibody, mogamulizumab, in combination with nivolumab in patients with advanced or metastatic solid tumors. Clin. Cancer Res. 2019, 25, 6614–6622. [Google Scholar] [CrossRef] [Green Version]
- Hong, D.S.; Rasco, D.; Veeder, M.; Luke, J.J.; Chandler, J.; Balmanoukian, A.; George, T.J.; Munster, P.; Berlin, J.D.; Gutierrez, M.; et al. A Phase 1b/2 Study of the Bruton Tyrosine Kinase Inhibitor Ibrutinib and the PD-L1 Inhibitor Durvalumab in Patients with Pretreated Solid Tumors. Oncology 2019, 97, 102–111. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.M.; Oh, D.-Y.; Dhani, N.; Renouf, D.J.; Lee, M.A.; Sun, W.; Fisher, G.; Hezel, A.; Chang, S.-H.; Vlahovic, G.; et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: A phase 2 randomized clinical trial. JAMA Oncol. 2019, 5, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Bockorny, B.; Semenisty, V.; Macarulla, T.; Borazanci, E.; Wolpin, B.M.; Stemmer, S.M.; Golan, T.; Geva, R.; Borad, M.J.; Pedersen, K.S.; et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: The COMBAT trial. Nat. Med. 2020, 26, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, D.; Wilkinson, G.A.; Eng, K.H.; Fields, P.; Raber, P.; Moseley, J.L.; Cheetham, K.; Coffey, M.; Nuovo, G.; Kalinski, P.; et al. Pembrolizumab in Combination with the Oncolytic Virus Pelareorep and Chemotherapy in Patients with Advanced Pancreatic Adenocarcinoma: A Phase Ib Study. Clin. Cancer Res. 2019, 26, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef]
- Le, D.T.; Lutz, E.; Uram, J.N.; Sugar, E.A.; Onners, B.; Solt, S.; Zheng, L.; Diaz, L.A.; Donehower, R.C.; Jaffee, E.M.; et al. Evaluation of Ipilimumab in Combination with Allogeneic Pancreatic Tumor Cells Transfected with a GM-CSF Gene in Previously Treated Pancreatic Cancer. J. Immunother. 2013, 36, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Aglietta, M.; Barone, C.; Sawyer, M.B.; Moore, M.J.; Miller, W.H., Jr.; Bagalà, C.; Colombi, F.; Cagnazzo, C.; Gioeni, L.; Wang, E.; et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann. Oncol. 2014, 25, 1750–1755. [Google Scholar] [CrossRef]
- Mohindra, N.A.; Kircher, S.M.; Nimeiri, H.S.; Benson, A.B.; Rademaker, A.; Alonso, E.; Blatner, N.; Khazaie, K.; Mulcahy, M.F. Results of the phase Ib study of ipilimumab and gemcitabine for advanced pancreas cancer. J. Clin. Oncol. 2015, 33, e15281. [Google Scholar] [CrossRef]
- Kalyan, A.; Kircher, S.M.; Mohindra, N.A.; Nimeiri, H.S.; Maurer, V.; Rademaker, A.; Benson, A.B.; Mulcahy, M.F. Ipilimumab and gemcitabine for advanced pancreas cancer: A phase Ib study. J. Clin. Oncol. 2016, 34, e15747. [Google Scholar] [CrossRef]
- Kamath, S.D.; Kalyan, A.; Kircher, S.; Nimeiri, H.; Fought, A.J.; Benson, A.; Mulcahy, M. Ipilimumab and Gemcitabine for Advanced Pancreatic Cancer: A Phase Ib Study. Oncol. 2019, 25, e808–e815. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, M.H.; O’Reilly, E.M.; Varadhachary, G.; Wolff, R.A.; Wainberg, Z.A.; Ko, A.H.; Fisher, G.; Rahma, O.; Lyman, J.P.; Cabanski, C.R.; et al. CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: An open-label, multicentre, phase 1b study. Lancet Oncol. 2021, 22, 118–131. [Google Scholar] [CrossRef]
- Zhen, D.B.; Whittle, M.; Ritch, P.S.; Hochster, H.S.; Coveler, A.L.; George, B.; Hendifar, A.E.; Dragovich, T.; Green, S.; Dion, B.; et al. Phase II study of PEGPH20 plus pembrolizumab for patients (pts) with hyaluronan (HA)-high refractory metastatic pancreatic adenocarcinoma (mPC): PCRT16-001. J. Clin. Oncol. 2022, 40, 576. [Google Scholar] [CrossRef]
- Emory University; Novartis; EUSA Pharma, Inc. Siltuximab and Spartalizumab in Patients with Metastatic Pancreatic Cancer. 2020. Available online: https://ClinicalTrials.gov/show/NCT04191421 (accessed on 6 May 2022).
- Massachusetts General Hospital; Bristol-Myers Squibb. Nivolumab and Ipilimumab and Radiation Therapy in MSS and MSI High Colorectal and Pancreatic Cancer. 2017. Available online: https://ClinicalTrials.gov/show/NCT03104439 (accessed on 6 May 2022).
- Massachusetts General Hospital; Bristol-Myers Squibb. Nivolumab + Ipilimumab + Radiation in MSS Pancreatic Cancer. 2020. Available online: https://ClinicalTrials.gov/show/NCT04361162 (accessed on 6 May 2022).
- University of Rochester; Syntrix Biosystems, Inc.; Bristol-Myers Squibb. A Study to Evaluate the Safety and Tolerability of SX-682 in Combination with Nivolumab as a Maintenance Therapy in Patients with Metastatic Pancreatic Ductal Adenocarcinoma. 2020. Available online: https://ClinicalTrials.gov/show/NCT04477343 (accessed on 6 May 2022).
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; Bristol-Myers Squibb. Pooled Mutant KRAS-Targeted Long Peptide Vaccine Combined with Nivolumab and Ipilimumab for Patients with Resected MMR-p Colorectal and Pancreatic Cancer. 2020. Available online: https://ClinicalTrials.gov/show/NCT04117087 (accessed on 6 May 2022).
- CanBas Co., Ltd. Study of CBP501/Cisplatin/Nivolumab Combinations in Advanced Pancreatic Cancer. 2021. Available online: https://ClinicalTrials.gov/show/NCT04953962 (accessed on 6 May 2022).
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; National Cancer Institute (NCI); Bristol-Myers Squibb. Platform Study of Neoadjuvant and Adjuvant Immunotherapy for Patients with Resectable Adenocarcinoma of the Pancreas. 2016. Available online: https://ClinicalTrials.gov/show/NCT02451982 (accessed on 6 May 2022).
- Jonsson Comprehensive Cancer Center; Bristol-Myers Squibb; NovoCure Ltd. Nivolumab in Combination with Chemotherapy Pre-Surgery in Treating Patients with Borderline Resectable Pancreatic Cancer. 2019. Available online: https://ClinicalTrials.gov/show/NCT03970252 (accessed on 6 May 2022).
- Massachusetts General Hospital; Bristol-Myers Squibb; Stand Up To Cancer; Lustgarten Foundation. Losartan and Nivolumab in Combination with FOLFIRINOX and SBRT in Localized Pancreatic Cancer. 2018. Available online: https://ClinicalTrials.gov/show/NCT03563248 (accessed on 6 May 2022).
- Manji, G.; Regeneron Pharmaceuticals; BioLine Rx. Chemo4METPANC Combination Chemokine Inhibitor, Immunotherapy, and Chemotherapy in Pancreatic Adenocarcinoma. 2021. Available online: https://ClinicalTrials.gov/show/NCT04543071 (accessed on 6 May 2022).
- National Cancer Institute (NCI). Testing the Combination of Anetumab Ravtansine with Either Nivolumab, Nivolumab and Ipilimumab, or Gemcitabine and Nivolumab in Advanced Pancreatic Cancer. 2019. Available online: https://ClinicalTrials.gov/show/NCT03816358 (accessed on 6 May 2022).
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; Bristol-Myers Squibb. Trial of Neoadjuvant and Adjuvant Nivolumab and BMS-813160 with or without GVAX for Locally Advanced Pancreatic Ductal Adenocarcinomas. 2019. Available online: https://ClinicalTrials.gov/show/NCT03767582 (accessed on 6 May 2022).
- Balachandran, V.P.; Beatty, G.L.; Dougan, S.K. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology 2019, 156, 2056–2072. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.V.; Park, S.; Phillips, P.A.; Santucci, N.; Goldstein, D.; Kumar, R.K.; Ramm, G.A.; Buchler, M.; Friess, H.; McCarroll, J.A.; et al. Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas 2004, 29, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Dougan, S.K. The Pancreatic Cancer Microenvironment. Cancer J. 2017, 23, 321–325. [Google Scholar] [CrossRef]
- Pothula, S.P.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Pancreatic stellate cells: Aiding and abetting pancreatic cancer progression. Pancreatology 2020, 20, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Ene–Obong, A.; Clear, A.J.; Watt, J.; Wang, J.; Fatah, R.; Riches, J.C.; Marshall, J.F.; Chin–Aleong, J.; Chelala, C.; Gribben, J.G.; et al. Activated Pancreatic Stellate Cells Sequester CD8+ T Cells to Reduce Their Infiltration of the Juxtatumoral Compartment of Pancreatic Ductal Adenocarcinoma. Gastroenterology 2013, 145, 1121–1132. [Google Scholar] [CrossRef] [Green Version]
- Lei, M.M.L.; Lee, T.K.W. Cancer Stem Cells: Emerging Key Players in Immune Evasion of Cancers. Front. Cell Dev. Biol. 2021, 9, 692940. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Shiota, G. Immune evasion by cancer stem cells. Regen. Ther. 2021, 17, 20–33. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, H.; Lee, D.-H.; Kim, T.-S.; Kim, T.; Chung, C.; Koh, G.Y.; Kim, H.; Lim, D.-S. Reversing the Intractable Nature of Pancreatic Cancer by Selectively Targeting ALDH-High, Therapy-Resistant Cancer Cells. PLoS ONE 2013, 8, e78130. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Petricoin, E.F.; Maitra, A.; Rajapakse, V.; King, C.; Jacobetz, M.A.; Ross, S.; Conrads, T.P.; Veenstra, T.D.; Hitt, B.A.; et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003, 4, 437–450. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhao, G.; Wu, W.; Rong, Y.; Jin, D.; Wang, D.; Lou, W.; Qin, X. Low intratumoral regulatory T cells and high peritumoral CD8+ T cells relate to long-term survival in patients with pancreatic ductal adenocarcinoma after pancreatectomy. Cancer Immunol. Immunother. 2015, 65, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Cui, M.; Yang, G.; Wang, H.; Feng, M.; You, L.; Zhao, Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol. Cancer 2018, 17, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieler, M.; Unseld, M.; Bianconi, D.; Prager, G. Challenges and Perspectives for Immunotherapy in Adenocarcinoma of the Pancreas: The Cancer Immunity Cycle. Pancreas 2018, 47, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Saka, D.; Gökalp, M.; Piyade, B.; Cevik, N.C.; Arik Sever, E.; Unutmaz, D.; Ceyhan, G.O.; Demir, I.E.; Asimgil, H. Mechanisms of T-Cell Exhaustion in Pancreatic Cancer. Cancers 2020, 12, 2274. [Google Scholar] [CrossRef]
- Whiting, C.; Lutz, E.; Nair, N.; Chang, S.; Lemmens, E.; Chen, S.Y.; Solt, S.; Ferber, S.; Maecker, H.; Murphy, A.; et al. Phase II, randomized study of GVAX pancreas and CRS-207 immunotherapy in patients with metastatic pancreatic cancer: Clinical update on long term survival and biomarker correlates to overall survival. J. Clin. Oncol. 2015, 33, 261. [Google Scholar] [CrossRef]
- Eric, L.; Yeo, C.J.; Lillemoe, K.D.; Biedrzycki, B.; Kobrin, B.; Herman, J.; Sugar, E.; Piantadosi, S.; Cameron, J.L.; Solt, S.; et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann. Surg. 2011, 253, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Le, D.T.; Picozzi, V.J.; Ko, A.H.; Wainberg, Z.A.; Kindler, H.; Wang-Gillam, A.; Oberstein, P.E.; Morse, M.A.; Zeh, H.J.; Weekes, C.D.; et al. Results from a Phase IIb, Randomized, Multicenter Study of GVAX Pancreas and CRS-207 Compared with Chemotherapy in Adults with Previously Treated Metastatic Pancreatic Adenocarcinoma (ECLIPSE Study). Clin. Cancer Res. 2019, 25, 5493–5502. [Google Scholar] [CrossRef]
- Middleton, G.; Silcocks, P.; Cox, T.; Valle, J.; Wadsley, J.; Propper, D.; Coxon, F.; Ross, P.; Madhusudan, S.; Roques, T.; et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): An open-label, randomised, phase 3 trial. Lancet Oncol. 2014, 15, 829–840. [Google Scholar] [CrossRef]
- Morrison, A.H.; Byrne, K.T.; Vonderheide, R.H. Immunotherapy and Prevention of Pancreatic Cancer. Trends Cancer 2018, 4, 418–428. [Google Scholar] [CrossRef]
- Thind, K.; Padrnos, L.J.; Ramanathan, R.K.; Borad, M.J. Immunotherapy in pancreatic cancer treatment: A new frontier. Ther. Adv. Gastroenterol. 2016, 10, 168–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V.; Greten, T.F.; Crocenzi, T.; Springett, G.; Morse, M.; Zeh, H.; Cohen, D.; Fine, R.L.; et al. Safety and Survival with GVAX Pancreas Prime and Listeria Monocytogenes–Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. J. Clin. Oncol. 2015, 33, 1325–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsujikawa, T.; Crocenzi, T.; Durham, J.N.; Sugar, E.A.; Wu, A.A.; Onners, B.; Nauroth, J.M.; Anders, R.A.; Fertig, E.J.; Laheru, D.A.; et al. Evaluation of Cyclophosphamide/GVAX Pancreas Followed by Listeria-Mesothelin (CRS-207) with or without Nivolumab in Patients with Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 3578–3588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, A.A.; Bever, K.M.; Ho, W.J.; Fertig, E.J.; Niu, N.; Zheng, L.; Parkinson, R.M.; Durham, J.N.; Onners, B.L.; Ferguson, A.K.; et al. A Phase II Study of Allogeneic GM-CSF–Transfected Pancreatic Tumor Vaccine (GVAX) with Ipilimumab as Maintenance Treatment for Metastatic Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 5129–5139. [Google Scholar] [CrossRef] [PubMed]
- Kaida, M.; Morita-Hoshi, Y.; Soeda, A.; Wakeda, T.; Yamaki, Y.; Kojima, Y.; Ueno, H.; Kondo, S.; Morizane, C.; Ikeda, M.; et al. Phase 1 Trial of Wilms Tumor 1 (WT1) Peptide Vaccine and Gemcitabine Combination Therapy in Patients with Advanced Pancreatic or Biliary Tract Cancer. J. Immunother. 2011, 34, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, S.; Koido, S.; Takeda, Y.; Homma, S.; Komita, H.; Takahara, A.; Morita, S.; Ito, T.; Morimoto, S.; Hara, K.; et al. Wilms Tumor Gene (WT1) Peptide–based Cancer Vaccine Combined with Gemcitabine for Patients with Advanced Pancreatic Cancer. J. Immunother. 2014, 37, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Koido, S.; Homma, S.; Okamoto, M.; Takakura, K.; Mori, M.; Yoshizaki, S.; Tsukinaga, S.; Odahara, S.; Koyama, S.; Imazu, H.; et al. Treatment with Chemotherapy and Dendritic Cells Pulsed with Multiple Wilms’ Tumor 1 (WT1)–Specific MHC Class I/II–Restricted Epitopes for Pancreatic Cancer. Clin. Cancer Res. 2014, 20, 4228–4239. [Google Scholar] [CrossRef] [Green Version]
- Tsukinaga, S.; Kajihara, M.; Takakura, K.; Ito, Z.; Kanai, T.; Saito, K.; Takami, S.; Kobayashi, H.; Matsumoto, Y.; Odahara, S.; et al. Prognostic significance of plasma interleukin-6/-8 in pancreatic cancer patients receiving chemoimmunotherapy. World J. Gastroenterol. WJG 2015, 21, 11168. [Google Scholar] [CrossRef]
- Mayanagi, S.; Kitago, M.; Sakurai, T.; Matsuda, T.; Fujita, T.; Higuchi, H.; Taguchi, J.; Takeuchi, H.; Itano, O.; Aiura, K.; et al. Phase I pilot study of Wilms tumor gene 1 peptide-pulsed dendritic cell vaccination combined with gemcitabine in pancreatic cancer. Cancer Sci. 2015, 106, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, R.; Koizumi, T.; Koya, T.; Sano, K.; Koido, S.; Nagai, K.; Kobayashi, M.; Okamoto, M.; Sugiyama, H.; Shimodaira, S. WT1-pulsed Dendritic Cell Vaccine Combined with Chemotherapy for Resected Pancreatic Cancer in a Phase I Study. Anticancer Res. 2018, 38, 2217–2225. [Google Scholar]
- Nishida, S.; Ishikawa, T.; Egawa, S.; Koido, S.; Yanagimoto, H.; Ishii, J.; Kanno, Y.; Kokura, S.; Yasuda, H.; Oba, M.S.; et al. Combination Gemcitabine and WT1 Peptide Vaccination Improves Progression-Free Survival in Advanced Pancreatic Ductal Adenocarcinoma: A Phase II Randomized Study. Cancer Immunol. Res. 2018, 6, 320–331. [Google Scholar] [CrossRef] [Green Version]
- Hanada, S.; Tsuruta, T.; Haraguchi, K.; Okamoto, M.; Sugiyama, H.; Koido, S. Long-term survival of pancreatic cancer patients treated with multimodal therapy combined with WT1-targeted dendritic cell vaccines. Hum. Vaccines Immunother. 2019, 15, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, K.; Adachi, T.; Harada, H.; Eguchi, S.; Sugiyama, H.; Miyazaki, Y. Dendritic Cell-based Immunotherapy Pulsed with Wilms Tumor 1 Peptide and Mucin 1 as an Adjuvant Therapy for Pancreatic Ductal Adenocarcinoma After Curative Resection: A Phase I/IIa Clinical Trial. Anticancer Res. 2020, 40, 5765–5776. [Google Scholar] [CrossRef] [PubMed]
- Asahara, S.; Takeda, K.; Yamao, K.; Maguchi, H.; Yamaue, H. Phase I/II clinical trial using HLA-A24-restricted peptide vaccine derived from KIF20A for patients with advanced pancreatic cancer. J. Transl. Med. 2013, 11, 291. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Hazama, S.; Ueno, T.; Matsui, H.; Shindo, Y.; Iida, M.; Yoshimura, K.; Yoshino, S.; Takeda, K.; Oka, M. A Phase I Clinical Trial of Vaccination with KIF20A-derived Peptide in Combination with Gemcitabine For Patients with Advanced Pancreatic Cancer. J. Immunother. 2014, 37, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazawa, M.; Ohsawa, R.; Tsunoda, T.; Hirono, S.; Kawai, M.; Tani, M.; Nakamura, Y.; Yamaue, H. Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci. 2010, 101, 433–439. [Google Scholar] [CrossRef]
- Yamaue, H.; Tsunoda, T.; Tani, M.; Miyazawa, M.; Yamao, K.; Mizuno, N.; Okusaka, T.; Ueno, H.; Boku, N.; Fukutomi, A.; et al. Randomized phase II / III clinical trial of elpamotide for patients with advanced pancreatic cancer: PEGASUS—PC Study. Cancer Sci. 2015, 106, 883–890. [Google Scholar] [CrossRef]
- Suzuki, N.; Hazama, S.; Iguchi, H.; Uesugi, K.; Tanaka, H.; Hirakawa, K.; Aruga, A.; Hatori, T.; Ishizaki, H.; Umeda, Y.; et al. Phase II clinical trial of peptide cocktail therapy for patients with advanced pancreatic cancer: VENUS-PC study. Cancer Sci. 2016, 108, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, M.; Katsuda, M.; Maguchi, H.; Katanuma, A.; Ishii, H.; Ozaka, M.; Yamao, K.; Imaoka, H.; Kawai, M.; Hirono, S.; et al. Phase II clinical trial using novel peptide cocktail vaccine as a postoperative adjuvant treatment for surgically resected pancreatic cancer patients. Int. J. Cancer 2017, 140, 973–982. [Google Scholar] [CrossRef]
- Kameshima, H.; Tsuruma, T.; Kutomi, G.; Shima, H.; Iwayama, Y.; Kimura, Y.; Imamura, M.; Torigoe, T.; Takahashi, A.; Hirohashi, Y.; et al. Immunotherapeutic benefit of α-interferon (IFNα) in survivin2B-derived peptide vaccination for advanced pancreatic cancer patients. Cancer Sci. 2012, 104, 124–129. [Google Scholar] [CrossRef]
- Shima, H.; Tsurita, G.; Wada, S.; Hirohashi, Y.; Yasui, H.; Hayashi, H.; Miyakoshi, T.; Watanabe, K.; Murai, A.; Asanuma, H.; et al. Randomized phase II trial of survivin 2B peptide vaccination for patients with HLA -A24-positive pancreatic adenocarcinoma. Cancer Sci. 2019, 110, 2378–2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Y.; Qin, X.; Jin, D.; Lou, W.; Wu, L.; Wang, D.; Wu, W.; Ni, X.; Mao, Z.; Kuang, T.; et al. A phase I pilot trial of MUC1-peptide-pulsed dendritic cells in the treatment of advanced pancreatic cancer. Clin. Exp. Med. 2011, 12, 173–180. [Google Scholar] [CrossRef]
- Le, D.T.; Brockstedt, D.G.; Nir-Paz, R.; Hampl, J.; Mathur, S.; Nemunaitis, J.; Sterman, D.H.; Hassan, R.; Lutz, E.; Moyer, B.; et al. A Live-Attenuated Listeria Vaccine (ANZ-100) and a Live-Attenuated Listeria Vaccine Expressing Mesothelin (CRS-207) for Advanced Cancers: Phase I Studies of Safety and Immune Induction. Clin. Cancer Res. 2012, 18, 858–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedén, S.; Klemp, M.; Gladhaug, I.P.; Møller, M.; Eriksen, J.A.; Gaudernack, G.; Buanes, T. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int. J. Cancer 2011, 128, 1120–1128. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Chapman, P.B.; Feilchenfeldt, J.; Brennan, M.F.; Capanu, M.; Gansukh, B.; Jacobs, G.; Levin, A.; Neville, D.; Kelsen, D.P.; et al. Targeting Mutated K-ras in Pancreatic Adenocarcinoma Using an Adjuvant Vaccine. Am. J. Clin. Oncol. 2011, 34, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Kubuschok, B.; Pfreundschuh, M.; Breit, R.; Hartmann, F.; Sester, M.; Gärtner, B.; König, J.; Murawski, N.; Held, G.; Zwick, C.; et al. Mutated Ras-Transfected, EBV-Transformed Lymphoblastoid Cell Lines as a Model Tumor Vaccine for Boosting T-Cell Responses Against Pancreatic Cancer: A Pilot Trial. Hum. Gene Ther. 2012, 23, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.H.; Valle, J.W.; Ma, Y.T.; Faluyi, O.; Neoptolemos, J.P.; Gjertsen, T.J.; Iversen, B.; Eriksen, J.A.; Møller, A.; Aksnes, A.; et al. TG01/GM-CSF and adjuvant gemcitabine in patients with resected RAS-mutant adenocarcinoma of the pancreas (CT TG01-01): A single-arm, phase 1/2 trial. Br. J. Cancer 2020, 122, 971–977. [Google Scholar] [CrossRef] [Green Version]
- Bassani-Sternberg, M.; Digklia, A.; Huber, F.; Wagner, D.; Sempoux, C.; Stevenson, B.J.; Thierry, A.; Michaux, J.; Pak, H.; Racle, J.; et al. A phase Ib study of the combination of personalized autologous dendritic cell vaccine, aspirin, and standard of care adjuvant chemotherapy followed by nivolumab for resected pancreatic adenocarcinoma—A proof of antigen discovery feasibility in three patients. Front Immunol. 2019, 10, 1832. [Google Scholar]
- Noguchi, M.; Yanagimoto, H.; Shiomi, H.; Satoi, S.; Mine, T.; Toyokawa, H.; Yamamoto, T.; Tani, T.; Yamada, A.; Kwon, A.-H.; et al. A phase II study of personalized peptide vaccination combined with gemcitabine for non-resectable pancreatic cancer patients. Oncol. Rep. 2010, 24, 795–801. [Google Scholar] [CrossRef]
- Bauer, C.; Dauer, M.; Saraj, S.; Schnurr, M.; Bauernfeind, F.; Sterzik, A.; Junkmann, J.; Jakl, V.; Kiefl, R.; Oduncu, F.; et al. Dendritic cell-based vaccination of patients with advanced pancreatic carcinoma: Results of a pilot study. Cancer Immunol. Immunother. 2011, 60, 1097–1107. [Google Scholar] [CrossRef]
- Kimura, Y.; Tsukada, J.; Tomoda, T.; Takahashi, H.; Imai, K.; Shimamura, K.; Sunamura, M.; Yonemitsu, Y.; Shimodaira, S.; Koido, S.; et al. Clinical and Immunologic Evaluation of Dendritic Cell–Based Immunotherapy in Combination with Gemcitabine and/or S-1 in Patients with Advanced Pancreatic Carcinoma. Pancreas 2012, 41, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Yutani, S.; Komatsu, N.; Yoshitomi, M.; Matsueda, S.; Yonemoto, K.; Mine, T.; Noguchi, M.; Ishihara, Y.; Yamada, A.; Itoh, K.; et al. A phase II study of a personalized peptide vaccination for chemotherapy-resistant advanced pancreatic cancer patients. Oncol. Rep. 2013, 30, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yun, M.M.; Xu, M.B.; Wang, Y.Z.; Yun, S. Pancreatic carcinoma-specific immunotherapy using synthesised alpha-galactosyl epitope-activated immune responders: Findings from a pilot study. Int. J. Clin. Oncol. 2012, 18, 657–665. [Google Scholar] [CrossRef]
- Lin, M.; Yuan, Y.-Y.; Liu, S.-P.; Shi, J.-J.; Long, X.-A.; Niu, L.-Z.; Chen, J.-B.; Li, Q.; Xu, K.-C. Prospective study of the safety and efficacy of a pancreatic cancer stem cell vaccine. J. Cancer Res. Clin. Oncol. 2015, 141, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Britten, C.D.; Chin, S.; Garrett-Mayer, E.; Cloud, C.A.; Li, M.; Scurti, G.; Salem, M.; Nelson, M.H.; Thomas, M.B.; et al. Vaccination with poly(IC:LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J. Hematol. Oncol. 2017, 10, 82. [Google Scholar] [CrossRef] [Green Version]
- Ota, S.; Miyashita, M.; Yamagishi, Y.; Ogasawara, M. Baseline immunity predicts prognosis of pancreatic cancer patients treated with WT1 and/or MUC1 peptide-loaded dendritic cell vaccination and a standard chemotherapy. Hum. Vaccines Immunother. 2021, 17, 5563–5572. [Google Scholar] [CrossRef]
- Zheng, L.; Ding, D.; Edil, B.H.; Judkins, C.; Durham, J.N.; Thomas, D.L.; Bever, K.M.; Mo, G.; Solt, S.E.; Hoare, J.A.; et al. Vaccine-Induced Intratumoral Lymphoid Aggregates Correlate with Survival Following Treatment with a Neoadjuvant and Adjuvant Vaccine in Patients with Resectable Pancreatic Adenocarcinoma. Clin. Cancer Res. 2021, 27, 1278–1286. [Google Scholar] [CrossRef]
- Washington University School of Medicine; National Institutes of Health (NIH); National Cancer Institute (NCI). Neoantigen Peptide Vaccine Strategy in Pancreatic Cancer Patients Following Surgical Resection and Adjuvant Chemotherapy. 2020. Available online: https://ClinicalTrials.gov/show/NCT03956056 (accessed on 6 May 2022).
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; The Skip Viragh Foundation. A Trial of Boost Vaccinations of Pancreatic Tumor Cell Vaccine. 2010. Available online: https://ClinicalTrials.gov/show/NCT01088789 (accessed on 6 May 2022).
- University of Pennsylvania. DC Vaccine in Pancreatic Cancer. 2018. Available online: https://ClinicalTrials.gov/show/NCT03592888 (accessed on 6 May 2022).
- M.D. Anderson Cancer Center; National Cancer Institute (NCI). Personalized Peptide Vaccine in Treating Patients with Advanced Pancreatic Cancer or Colorectal Cancer. 2016. Available online: https://ClinicalTrials.gov/show/NCT02600949 (accessed on 6 May 2022).
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; Merck Sharp & Dohme LLC. Epacadostat, Pembrolizumab, and CRS-207, with or without CY/GVAX Pancreas in Patients with Metastatic Pancreas Cancer. 2018. Available online: https://ClinicalTrials.gov/show/NCT03006302 (accessed on 6 May 2022).
- Baylor College of Medicine; Cancer Cures for Kids. Th-1 Dendritic Cell Immunotherapy Plus Standard Chemotherapy for Pancreatic Adenocarcinoma. 2020. Available online: https://ClinicalTrials.gov/show/NCT04157127 (accessed on 6 May 2022).
- Varghese, A.M. Chimeric antigen receptor (CAR) T and other T cell strategies for pancreas adenocarcinoma. Chin. Clin. Oncol. 2017, 6, 66. [Google Scholar] [CrossRef]
- Smaglo, B.G.; Musher, B.L.; Vasileiou, S.; Kuvalekar, M.; Watanabe, A.; Robertson, C.; Wang, T.; Francois, M.; Ramos, C.A.; Hill, L.; et al. A phase I trial targeting advanced or metastatic pancreatic cancer using a combination of standard chemotherapy and adoptively transferred nonengineered, multiantigen specific T cells in the first-line setting (TACTOPS). J. Clin. Oncol. 2020, 38, 4622. [Google Scholar] [CrossRef]
- Biasci, D.; Smoragiewicz, M.; Connell, C.M.; Wang, Z.; Gao, Y.; Thaventhiran, J.E.D.; Basu, B.; Magiera, L.; Johnson, T.I.; Bax, L.; et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc. Natl. Acad. Sci. USA 2020, 117, 28960–28970. [Google Scholar] [CrossRef]
- O’Hara, M.H.; O’Reilly, E.M.; Wolff, R.A.; Wainberg, Z.A.; Ko, A.H.; Rahma, O.E.; Fisher, G.A.; Lyman, J.P.; Cabanski, C.R.; Karakunnel, J.J.; et al. Gemcitabine (Gem) and nab-paclitaxel (NP) ± nivolumab (nivo) ± CD40 agonistic monoclonal antibody APX005M (sotigalimab), in patients (Pts) with untreated metastatic pancreatic adenocarcinoma (mPDAC): Phase (Ph) 2 final results. J. Clin. Oncol. 2021, 39, 4019. [Google Scholar] [CrossRef]
- Byrne, K.T.; Betts, C.B.; Mick, R.; Sivagnanam, S.; Bajor, D.L.; Laheru, D.A.; Chiorean, E.G.; O’Hara, M.H.; Liudahl, S.M.; Newcomb, C.W.; et al. Neoadjuvant Selicrelumab, an Agonist CD40 Antibody, Induces Changes in the Tumor Microenvironment in Patients with Resectable Pancreatic Cancer. Clin. Cancer Res. 2021, 27, 4574–4586. [Google Scholar] [CrossRef] [PubMed]
- Perkhofer, L.; Gout, J.; Roger, E.; de Almeida, F.K.; Simões, C.B.; Wiesmüller, L.; Seufferlein, T.; Kleger, A. DNA damage repair as a target in pancreatic cancer: State-of-the-art and future perspectives. Gut 2021, 70, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Casolino, R.; Paiella, S.; Azzolina, D.; Beer, P.A.; Corbo, V.; Lorenzoni, G.; Gregori, D.; Golan, T.; Braconi, C.; Froeling, F.E.M.; et al. Homologous Recombination Deficiency in Pancreatic Cancer: A Systematic Review and Prevalence Meta-Analysis. J. Clin. Oncol. 2021, 39, 2617–2631. [Google Scholar] [CrossRef]
- Heeke, A.L.; Pishvaian, M.J.; Lynce, F.; Xiu, J.; Brody, J.R.; Chen, W.-J.; Baker, T.M.; Marshall, J.L.; Isaacs, C. Prevalence of Homologous Recombination–Related Gene Mutations Across Multiple Cancer Types. JCO Precis. Oncol. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Blais, E.M.; Brody, J.R.; Rahib, L.; Lyons, E.; De Arbeloa, P.; Hendifar, A.; Mikhail, S.; Chung, V.; Sohal, D.P.; et al. Outcomes in Patients with Pancreatic Adenocarcinoma with Genetic Mutations in DNA Damage Response Pathways: Results From the Know Your Tumor Program. JCO Precis. Oncol. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Wattenberg, M.M.; Reiss, K.A. Determinants of Homologous Recombination Deficiency in Pancreatic Cancer. Cancers 2021, 13, 4716. [Google Scholar] [CrossRef]
- Mittica, G.; Ghisoni, E.; Giannone, G.; Genta, S.; Aglietta, M.; Sapino, A.; Valabrega, G. PARP Inhibitors in Ovarian Cancer. Recent Patents Anti-Cancer Drug Discov. 2018, 13, 392–410. [Google Scholar] [CrossRef]
- Kim, D.-S.; Camacho, C.V.; Kraus, W.L. Alternate therapeutic pathways for PARP inhibitors and potential mechanisms of resistance. Exp. Mol. Med. 2021, 53, 42–51. [Google Scholar] [CrossRef]
- D’Andrea, A.D. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair 2018, 71, 172–176. [Google Scholar] [CrossRef]
- Caron, M.-C.; Sharma, A.K.; O’Sullivan, J.; Myler, L.R.; Ferreira, M.T.; Rodrigue, A.; Coulombe, Y.; Ethier, C.; Gagné, J.-P.; Langelier, M.-F.; et al. Poly(ADP-ribose) polymerase-1 antagonizes DNA resection at double-strand breaks. Nat. Commun. 2019, 10, 2954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- Zhu, H.; Wei, M.; Xu, J.; Hua, J.; Liang, C.; Meng, Q.; Zhang, Y.; Liu, J.; Zhang, B.; Yu, X.; et al. PARP inhibitors in pancreatic cancer: Molecular mechanisms and clinical applications. Mol. Cancer 2020, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Pishvaian, M.J.; Hwang, J.J.; He, A.R.; Smaglo, B.G.; Kim, S.S.; Weinberg, B.A.; Weiner, L.M.; Marshall, J.L.; Brody, J.R. A Phase I/II Study of Veliparib (ABT-888) in Combination with 5-Fluorouracil and Oxaliplatin in Patients with Metastatic Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 5092–5101. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.M.; Lee, J.W.; Zalupski, M.; Capanu, M.; Park, J.; Golan, T.; Tahover, E.; Lowery, M.A.; Chou, J.F.; Sahai, V.; et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin with or without Veliparib in Patients with Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J. Clin. Oncol. 2020, 38, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Chiorean, E.G.; Guthrie, K.A.; Philip, P.A.; Swisher, E.M.; Jalikis, F.; Pishvaian, M.J.; Berlin, J.; Noel, M.S.; Suga, J.M.; Garrido-Laguna, I.; et al. Randomized Phase II Study of PARP Inhibitor ABT-888 (Veliparib) with Modified FOLFIRI versus FOLFIRI as Second-line Treatment of Metastatic Pancreatic Cancer: SWOG S1513. Clin. Cancer Res. 2021, 27, 6314–6322. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Myzak, M.C.; Johnson, B.A.; De Jesus-Acosta, A.; Le, D.T.; Jaffee, E.; Azad, N.S.; Donehower, R.C.; Zheng, L.; Oberstein, P.E.; et al. Olaparib in combination with irinotecan, cisplatin, and mitomycin C in patients with advanced pancreatic cancer. Oncotarget 2017, 8, 44073–44081. [Google Scholar] [CrossRef]
- Toh, M.; Ngeow, J. Homologous Recombination Deficiency: Cancer Predispositions and Treatment Implications. Oncologist 2021, 26, e1526–e1537. [Google Scholar] [CrossRef]
- Marshall, C.H.; Sokolova, A.O.; McNatty, A.L.; Cheng, H.H.; Eisenberger, M.A.; Bryce, A.H.; Schweizer, M.T.; Antonarakis, E.S. Differential Response to Olaparib Treatment Among Men with Metastatic Castration-resistant Prostate Cancer Harboring BRCA1 or BRCA2 Versus ATM Mutations. Eur. Urol. 2019, 76, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.; Wood, A.C.; Yu, J.; Kim, R. Investigational PARP inhibitors for the treatment of biliary tract cancer: Spotlight on preclinical and clinical studies. Expert Opin. Investig. Drugs 2021, 30, 451–461. [Google Scholar] [CrossRef]
- Higuchi, T.; Flies, D.B.; Marjon, N.A.; Mantia-Smaldone, G.; Ronner, L.; Gimotty, P.A.; Adams, S.F. CTLA-4 Blockade Synergizes Therapeutically with PARP Inhibition in BRCA1-Deficient Ovarian Cancer. Cancer Immunol. Res. 2015, 3, 1257–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, S.; Xia, W.; Yamaguchi, H.; Wei, Y.; Chen, M.K.; Hsu, J.M.; Hsu, J.L.; Yu, W.H.; Du, Y.; Lee, H.H.; et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin. Cancer Res. 2017, 23, 3711–3720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, B.; Pan, W.; Xing, Y.; Xiao, Y.; Chen, J.; Xu, Z. Recent advances in DDR (DNA damage response) inhibitors for cancer therapy. Eur. J. Med. Chem. 2022, 230, 114109. [Google Scholar] [CrossRef] [PubMed]
- Hechtman, J.F. NTRK insights: Best practices for pathologists. Mod. Pathol. 2022, 35, 298–305. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Allen, M.J.; Zhang, A.; Bavi, P.; Kim, J.C.; Jang, G.H.; Kelly, D.; Perera, S.; Denroche, R.E.; Notta, F.; Wilson, J.M.; et al. Molecular characterisation of pancreatic ductal adenocarcinoma with NTRK fusions and review of the literature. J. Clin. Pathol. 2021. [Google Scholar] [CrossRef]
- Cook, J.H.; Melloni, G.E.M.; Gulhan, D.C.; Park, P.J.; Haigis, K.M. The origins and genetic interactions of KRAS mutations are allele- and tissue-specific. Nat. Commun. 2021, 12, 1808. [Google Scholar] [CrossRef]
- Bannoura, S.F.; Uddin, H.; Nagasaka, M.; Fazili, F.; Al-Hallak, M.N.; Philip, P.A.; El-Rayes, B.; Azmi, A.S. Targeting KRAS in pancreatic cancer: New drugs on the horizon. Cancer Metastasis Rev. 2021, 40, 819–835. [Google Scholar] [CrossRef]
- Moore, A.R.; Rosenberg, S.C.; McCormick, F.; Malek, S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020, 19, 533–552. [Google Scholar] [CrossRef]
- Ostrem, J.M.; Peters, U.; Sos, M.L.; Wells, J.A.; Shokat, K.M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 2013, 503, 548–551. [Google Scholar] [CrossRef] [Green Version]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.S.; Spira, A.I.; Yaeger, R.; Buchschacher, G.L.; McRee, A.J.; Sabari, J.K.; Johnson, M.L.; Barve, M.A.; Hafez, N.; Velastegui, K.; et al. KRYSTAL-1: Updated activity and safety of adagrasib (MRTX849) in patients (Pts) with unresectable or metastatic pancreatic cancer (PDAC) and other gastrointestinal (GI) tumors harboring a KRASG12C mutation. J. Clin. Oncol. 2022, 40, 519. [Google Scholar] [CrossRef]
- Tanaka, N.; Lin, J.J.; Li, C.; Ryan, M.B.; Zhang, J.; Kiedrowski, L.A.; Michel, A.G.; Syed, M.U.; Fella, K.A.; Sakhi, M.; et al. Clinical Acquired Resistance to KRASG12C Inhibition through a Novel KRAS Switch-II Pocket Mutation and Polyclonal Alterations Converging on RAS–MAPK Reactivation. Cancer Discov. 2021, 11, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.R.; Somer, B.G.; Park, J.O.; Li, C.-P.; Scheulen, M.E.; Kasubhai, S.M.; Oh, D.-Y.; Liu, Y.; Redhu, S.; Steplewski, K.; et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur. J. Cancer 2014, 50, 2072–2081. [Google Scholar] [CrossRef]
- Bodoky, G.; Timcheva, C.; Spigel, D.R.; La Stella, P.J.; Ciuleanu, T.E.; Pover, G.; Tebbutt, N.C. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Investig. New Drugs 2011, 30, 1216–1223. [Google Scholar] [CrossRef]
- Xavier, C.B.; Marchetti, K.R.; Castria, T.B.; Jardim, D.L.F.; Fernandes, G.S. Trametinib and Hydroxychloroquine (HCQ) Combination Treatment in KRAS-Mutated Advanced Pancreatic Adenocarcinoma: Detailed Description of Two Cases. J. Gastrointest. Cancer 2020, 52, 374–380. [Google Scholar] [CrossRef]
- Colak, S.; ten Dijke, P. Targeting TGF-β Signaling in Cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Principe, D.R.; Park, A.; Dorman, M.J.; Kumar, S.; Viswakarma, N.; Rubin, J.; Torres, C.; McKinney, R.; Munshi, H.G.; Grippo, P.J.; et al. TGFβ Blockade Augments PD-1 Inhibition to Promote T-Cell–Mediated Regression of Pancreatic Cancer. Mol. Cancer Ther. 2019, 18, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Luengo, A.; Gui, D.Y.; Vander Heiden, M.G. Targeting Metabolism for Cancer Therapy. Cell Chem. Biol. 2017, 24, 1161–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, J.; Lyssiotis, C.A.; Ying, H.; Wang, X.; Hua, S.; Ligorio, M.; Perera, R.M.; Ferrone, C.R.; Mullarky, E.; Shyh-Chang, N.; et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013, 496, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Hammel, P.; Fabienne, P.; Mineur, L.; Metges, J.-P.; Andre, T.; De La Fouchardiere, C.; Louvet, C.; El Hajbi, F.; Faroux, R.; Guimbaud, R.; et al. Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: An open-label, randomized Phase IIb trial. Eur. J. Cancer 2020, 124, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Darmanin, S.; Natsuisaka, M.; Kondo, T.; Asaka, M.; Shindoh, M.; Higashino, F.; Hamuro, J.; Okada, F.; Kobayashi, M.; et al. Enhanced Expression of Asparagine Synthetase under Glucose-Deprived Conditions Protects Pancreatic Cancer Cells from Apoptosis Induced by Glucose Deprivation and Cisplatin. Cancer Res. 2007, 67, 3345–3355. [Google Scholar] [CrossRef] [Green Version]
- Hammel, P.; El-Hariry, I.; Macarulla, T.; Garcia-Carbonero, R.; Metges, J.-P.; Bouché, O.; Portales, F.; Cid, R.A.P.; Mineur, L.; Gracian, A.M.C.; et al. Trybeca-1: A randomized, phase 3 study of eryaspase in combination with chemotherapy versus chemotherapy alone as second-line treatment in patients with advanced pancreatic adenocarcinoma (NCT03665441). J. Clin. Oncol. 2022, 40, 518. [Google Scholar] [CrossRef]
- Yin, C.; Marshall, J.; Macke, L.; Bouker, K.; He, A.R.; Weinberg, B.A.; Weiner, L.M.; Wang, H.; Badri, N.; Biswas-Baldwin, N.; et al. A phase I dose-escalation study of eryaspase in combination with modified FOLFIRINOX in locally advanced and metastatic pancreatic ductal adenocarcinoma: Interim update. J. Clin. Oncol. 2022, 40, 581. [Google Scholar] [CrossRef]
- Xu, R.; Yang, J.; Ren, B.; Wang, H.; Yang, G.; Chen, Y.; You, L.; Zhao, Y. Reprogramming of Amino Acid Metabolism in Pancreatic Cancer: Recent Advances and Therapeutic Strategies. Front. Oncol. 2020, 10, 572722. [Google Scholar] [CrossRef]
- Chakrabarti, G.; Moore, Z.R.; Luo, X.; Ilcheva, M.; Ali, A.; Padanad, M.; Zhou, Y.; Xie, Y.; Burma, S.; Scaglioni, P.P.; et al. Targeting glutamine metabolism sensitizes pancreatic cancer to PARP-driven metabolic catastrophe induced by ß-lapachone. Cancer Metab. 2015, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Bott, A.J.; Shen, J.; Tonelli, C.; Zhan, L.; Sivaram, N.; Jiang, Y.-P.; Yu, X.; Bhatt, V.; Chiles, E.; Zhong, H.; et al. Glutamine Anabolism Plays a Critical Role in Pancreatic Cancer by Coupling Carbon and Nitrogen Metabolism. Cell Rep. 2019, 29, 1287–1298.e6. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Pu, N.; Yin, H.; Zhang, J.; Zhao, G.; Lou, W.; Wu, W. CD73 acts as a prognostic biomarker and promotes progression and immune escape in pancreatic cancer. J. Cell. Mol. Med. 2020, 24, 8674–8686. [Google Scholar] [CrossRef]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. Immunity, inflammation and cancer: A leading role for adenosine. Nat. Cancer 2013, 13, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Kshirsagar, P.G.; Gautam, S.K.; Gulati, M.; Wafa, E.I.; Christiansen, J.C.; White, B.M.; Mallapragada, S.K.; Wannemuehler, M.J.; Kumar, S.; et al. Nanocarriers for pancreatic cancer imaging, treatments, and immunotherapies. Theranostics 2022, 12, 1030–1060. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Population | Trial Phase, Year, Author, Ref. | Number of Patients | mPFS (Months) | mOS (Months) | Results |
|---|---|---|---|---|---|---|
| (1) Durvalumab 1500 mg + galunisertib 50 mg 1×/day | mPDAC | Phase Ib, 2021, Melisi, [17] | (1) 3 | (1) NR | (1) NR | 15/32 patient had PD, and DCR was 25.0%. |
| (2) Durvalumab 1500 mg + galunisertib 50 mg 2×/day | (2) 4 | (2) NR | (2) NR | |||
| (3) Durvalumab 1500 mg + galunisertib 80 mg 2×/day | (3) 3 | (3) NR | (3) NR | |||
| (4) Durvalumab 1500 mg + galunisertib 150 mg 2×/day | (4) 32 | (4) 1.8 | (4) 1.8 | |||
| Nivolumab + ipilimumab + radiation | mPDAC | Phase II, 2021, Parikh, [18] | 25 | 2.5 | 4.2 | DCR was 20% (5/25) of PDAC patients. |
| Anti-PD-L1 | Pre-treated LAPC/mPDAC | Phase I, 2012, Brahmer, [19] | 14 | NR | NR | No objective responses seen in patients with PDAC. |
| Pembrolizumab + multiple chemo arms | Pre-treated mPDAC | Phase 1b, 2017, Weiss, [20] | 11 | NR | 8 | No additional data reported for PDAC. |
| Pembrolizumab + GemNab | Pre-treated and untreated mPDAC | Phase Ib-II, 2018, Weiss, [21] | 17 | 9.1 | 15 | DCR was 100% in 11 chemo naïve PDAC patients. |
| Nivolumab + mogamulizumab | Pre-treated mPDAC | Phase I, 2019, Doi, [22] | 15 | 1.8 | 6.5 | DCR was 40% (6/15) and ORR seen in 1/15 patient with PDAC. |
| Durvalumab + ibrutinib | Pre-treated LAPC/mPDAC | Phase Ib-II, 2019, Hong, [23] | 49 | 1.7 | 4.2 | ORR seen in 2% of patients with PDAC. |
| Durvalumab (D) + tremelimumab (T) or durvalumab (D) monotherapy | Pre-treated mPDAC | Phase II, 2019, O’Reilly, [24] | 65 | 9.4 (D+T) 3.6 (D) | 8.8 (D+T) 6.3 (D) | Combination treatment resulted in an ORR of 3.1%, while monotherapy resulted in an ORR of 0%. |
| (1) Anti-CXCR4 + pembrolizumab (2) Anti-CXCR4 + pembrolizumab + chemo | Pre-treated mPDAC | Phase IIa, 2020, Bockorny, [25] | 59 | NR | (1) 3.3 (2) 7.2 | DCR was 34.5% in patient treated with anti-CXCR4 + Pembrolizumab and 32% in patient with combination of anti-CXCR4 and pembrolizumab with chemotherapy. |
| Pembrolizumab | Pre-treated MSI-H LAPC/mPDAC | Phase II, 2020, Marabelle, [11] | 22 | 2.1 | 4.0 | mDOR was 13.4 months in patients with PDAC. |
| Pembrolizumab + oncolytic virus (Pelareorep) + chemo | Pre-treated LAPC/mPDAC | Phase Ib, 2020, Mahalingam, [26] | 11 | 2 | 3.1 | The ORR and DCR were, respectively, 9% and 27%. |
| Ipilimumab | Pre-treated LAPC/mPDAC | Phase Ib, 2010, Royal, [27] | 27 | NR | NR | No responders to single agent Ipilimumab observed. |
| Ipilimumab (1) Monotherapy (2) + GVAX | Pre-treated LAPC/mPDAC | Phase Ib, 2013, Le, [28] | 30 | NR | (1) 3.6 (2) 5.7 | 3 patients in combination arm had prolonged SD. 2 patients in monotherapy arm had SD |
| Tremelimumab + gemcitabine | chemo naïve mPDAC | Phase Ib, 2014, Aglietta, [29] | 34 | NR | 7.4 | 2 patients had PR. |
| Ipilimumab + gemcitabine | Previously treated LAPC/mPDAC | Phase Ib, 2015, Mohindra, [30] | 13 | NR | NR | PR was seen in 2 pts (15%) and stable disease in 5 pts (38%). |
| Ipilimumab + gemcitabine | Pre-treated mPDAC | Phase Ib, 2016, Kaylan, [31] | 16 | 2.5 | 8.3 | The ORR was 14% (3/21), and seven patients had SD. |
| Ipilimumab + gemcitabine | Pre-treated mPDAC | Phase Ib, 2020, Kamath, [32] | 21 | 2.78 | 6.90 | PR seen in 2/16 patients and SD seen 5/16 patients. |
| (1) Nivolumab + GemNab + APX005M (0.1 mg/m2) | mPDAC | Phase Ib, 2021, O’Hara, [33] | (1) 6 | (1) 10.8 | (1) 15.9 | ORR 58% (14 patients). |
| (2) Nivolumab + GemNab + APX005M (0.3 mg/m2) | (2) 6 | (2) 12.4 | (2) NR | |||
| (3) GemNab + APX005M (0.1 mg/m2) | (3) 6 | (3) 12.5 | (3) 12.7 | |||
| (4) GemNab + APX005M (0.3 mg/m2) | (4) 6 | (4) 10.4 | (4) 20.1 | |||
| Pegvorhyaluronidase alfa (PEGPH20) + pembrolizumab | Pre-treated mPDAC | Phase II, 2022, Zhen, [34] | 38 | 1.5 | 7.2 | SD in 2 patients (25%), lasting 2.2 and 9 months. |
| Trial Reference | Phase | Treatment | Population | Number of Patients |
|---|---|---|---|---|
| NCT04191421 [35] | Ib-II | Spartalizumab + siltuximab | mPDAC | 42 |
| NCT03104439 [36] | II | Nivolumab + ipilimumab + radiation | PDAC | 80 |
| NCT04361162 [37] | II | Ipilimumab + nivolumab + radiation therapy | mPDAC | 30 |
| NCT04477343 [38] | I | SX-682 + nivolumab | mPDAC | 20 |
| NCT04117087 [39] | I | KRAS peptide vaccine + nivolumab + ipilimumab | Resected MMR-p Colorectal cancer and PDAC | 30 |
| NCT04953962 [40] | II | CBP501 + cisplatin + nivolumab | mPDAC | 92 |
| NCT02451982 [41] | II | Arm A: CY/GVAX Arm B: CY/GVAX + nivolumab Arm C: CY/GVAX + nivolumab + urelumab Arm D: BMS-986253 + nivolumab | Surgically resectable PDAC | 76 |
| NCT03970252 [42] | I | Nivolumab, mFOLFIRINOX | Borderline resectable PDAC | 36 |
| NCT03563248 [43] | II |
| LAPC | 160 |
| NCT04543071 [44] | II | Motixafortide, cemiplimab, gemcitabine, nab-paclitaxel | PDAC | 10 |
| NCT03816358 [45] | I-II |
| Mesothelin-positive PDAC | 74 |
| NCT03767582 [46] | I-II | Phase I: GVAX + nivolumab + CCR2/CCR5 Phase II: (1) nivolumab + CCR2/CCR5 (2) Nivolumab + GVAX + CCR2/CCR5 | LAPC | 30 |
| Trial Phase, Author, Year, Ref. | Patient Population | Treatment | Vaccine Type; Vaccine Route | Number of Patients | mPFS (Months) | mOS (Months) |
|---|---|---|---|---|---|---|
| Phase II, Lutz, 2011, [61] | Resected PDAC | GVAX (+ GM-CSF) + resection + CRT | Whole-tumor-cell; ID | 60 | 17.3 | 24.8 |
| Phase II, Le, 2015, [66] | Pre-treated mPDAC |
| Whole-tumor-cell; ID | 90 | NR | (1) 6.1 (2) 3.9 |
| Phase IIb, Le, 2019, [62] | Pre-treated mPDAC |
| Whole-tumor-cell; ID | 169 | (1) 2.3 (2) 2.1 (3) 2.1 | (1) 3.7 (2) 5.4 (3) 4.6 |
| Phase II, Tsujikawa, 2020, [67] | Pre-treated mPDAC |
| Whole-tumor-cell; ID | 93 | (1) 2.2 (2) 2.2 | (1) 5.9 (t) (2) 6.1 (t) |
| Phase II, Wu, 2020, [68] | Pre-treated mPDAC |
| Whole-tumor-cell; ID | 82 | (1) 2.4 (2) 5.6 | (1) 9.4 (2) 14.7 |
| Phase I, Kaida, 2011, [69] | Gemcitabine-naïve LAPC/mPDAC | WT-1 vaccine + gemcitabine | Peptide; ID | 9 | NR | 8.2 |
| Phase I, Nishida, 2014, [70] | Untreated LAPC/mPDAC and treated recurrent disease | WT-1 vaccine + gemcitabine | Peptide; ID | 32 | 4.2 | 8.1 |
| Phase I, Koido, 2014, [71] | mPDAC: untreated newly diagnosed or recurrence after resection | WT-1 vaccine + gemcitabine | Peptide; ID | 10 | NR | NR |
| NR, Tsukinaga, 2015, [72] | Untreated mPDAC | WT-1 vaccine + gemcitabine | DC; ID | 7 | 6.8 | 10.7 |
| Phase I, Mayanagi, 2015, [73] | Treatment-naïve LAPC/mPDAC | WT-1 vaccine + gemcitabine | DC; ID | 10 | NR | 8 |
| Phase I, Yanagisawa, 2018, [74] | Resected, chemo-naïve PDAC | WT-1 vaccine + chemo | DC; ID | 8 | NR | NR |
| Phase II, Nishida, 2018, [75] | Untreated LAPC, mPDAC, or recurrence after resection |
| Peptide; ID | 85 | (1) 5.2 (2) 3.3 | (1) 9.6 (2) 8.9 |
| NR, Hanada, 2020, [76] | Pre-resected recurrent PDAC | WT-1 vaccine | DC; ID | 6 | 19.9 | 59 |
| Phase I-IIa, Nagai, 2020, [77] | Pre-resected PDAC | WT-1/MUC-1 vaccine + gemcitabine | DC; ID | 10 | 17.7 | 46.4 |
| Phase I-II, Asahara, 2013, [78] | Chemo-refractory, LAPC/mPDAC, or recurrence after resection |
| Peptide; SC | 110 | (1) 1.8 (2) NR | (1) 4.7 (2) 2.1 |
| Phase I, Suzuki, 2014, [79] | Pre-treated LAPC/mPDAC | KIF20A vaccine + gemcitabine | Peptide; SC | 9 | NR | 57 |
| Phase I, Miyazawa, 2010, [80] | LAPC/mPDAC | VEGFR2 vaccine + gemcitabine | Peptide; SC | 18 | 3.9 | 7.7 |
| Phase II-III, Yamaue, 2015, [81] | Untreated LAPC/mPDAC |
| Peptide; SC | 153 | (1) 3.7 (2) 3.8 | (1) 8.4 (2) 8.5 |
| Phase II, Suzuki, 2017, [82] | Untreated LAPC/mPDAC | KIF20A + VEGFR1/2 vaccine + gemcitabine | Peptide; SC | 68 | 4.7–5.2 | 9–10 |
| Phase II, Miyazawa, 2017, [83] | Pre-resected PDAC | KIF20A + VEGFR1/2 vaccine + gem | Peptide; SC | 30 | 15.8 | NR |
| NR, Kameshima, 2013, [84] | LAPC/mPDAC | Survivin vaccine + IFA, IFNα | Peptide; SC | 6 | NR | NR |
| Phase II, Shima, 2019, [85] | Pre-treated LAPC/mPDAC |
| Peptide; SC | 83 | (1) 2.2 (3) 2.3 | (1) 3.4 (t) (2) 3.2 (t) (3) 3.6 (t) |
| Phase I, Rong, 2012, [86] | Pre-treated LAPC/mPDAC | MUC-1 vaccine | DC; ID | 6 | NR | NR |
| Phase I, Le, 2012, [87] | Pre-treated PDAC | Mesothelin expressing Lm Vaccine | Lm; IV | 9 | NR | 7 |
| Phase I, Middleton, 2014, [63] | Untreated LAPC/mPDAC |
| Peptide; ID | 1062 | (1) 6.4 (2) 4.5 (3) 6.6 | (1) 7.9 (2) 6.9 (3) 8.4 |
| Phase I-II, Wedén, 2011, [88] | Pre-resected PDAC | KRAS vaccine + GM-CSF | Peptide; ID | 23 | NR | 27.5 |
| NR, Abou-Alfa, 2011, [89] | Pre-resected PDAC | KRAS vaccine + GM-CSF | Peptide; ID | 24 | 8.6 | 20.3 |
| Phase I, Kubuschok, 2012, [90] | mPDAC | KRAS vaccine | LCL; SC | 7 | 3.1 | 4.5 |
| Phase I-II, Palmer, 2020, [91] | Pre-resected PDAC | KRAS vaccine + GM-CSF + gemcitabine | Peptide; ID | 32 | 13.9–19.5 | 33.1–34.2 |
| Phase Ib, Bassani- Sternberg, 2019, [92] | Pre-resected PDAC | Neoantigens + chemo + anti-PD-1 + aspirin | DC; SC | 3 | NR | NR |
| Phase II, Yanagimoto, 2010, [93] | Untreated LAPC/mPDAC | Personalized Vaccine + gemcitabine | Peptide; SC | 21 | 7 | 9 |
| Phase I, Bauer, 2011, [94] | Pre-resected recurrent PDAC | Tumor lysate Vaccine + gemcitabine | DC; ID | 12 | NR | 10.5 |
| NR, Kimura, 2012, [95] | Chemo-refractory LAPC/mPDAC | Personalized and/or tumor lysate vaccine + chemo + LAK cell therapy | DC; IT | 49 | NR | 11.8 |
| Phase II, Yutani, 2013, [96] | Chemo-refractory mPDAC, | Personalized vaccine + chemo | Peptide; SC | 41 | NR | 7.9 |
| Phase I, Qiu, 2013, [97] | Pre-treated LAPC/mPDAC | Tumor lysate expressing -Gal + CIK cell therapy | DC; ID | 14 | NR | 24.7 |
| NR, Lin, 2015, [98] | Pre-treated stage II PDAC, LAPC, mPDAC | Pancreatic cancer stem cell lysate | Whole-tumor-cell; SC | 90 | NR | NR |
| Phase I, Mehrotra, 2017, [99] | Pre-treated LAPC/mPDAC | hTERT, CEA, Survivin vaccine + TLR-3 agonist | DC-ID | 12 | 3 | 7.7 |
| Phase 1–11, Ota, 2021, [100] | Advanced or recurrent PDAC | WT1 and/or MUC1 + GEM plus nab-PTX or FOLFIRINOX regimen | Peptide-ID | 48 | 8.1 | 15.1 |
| Phase II, Zheng, 2021, [101] | Pre-resectable PDAC | GVAX + Cy | Whole tumor cell-ID | (1) 29 (2) 28 (3) 30 | (1) NR (2) NR (3) NR | (1) 34.2 (2) 15.4 (3) 16.5 |
| Trial Reference | Phase | Treatment | Population | Number of Patients |
|---|---|---|---|---|
| NCT03956056 [102] | I | Neoantigen peptide vaccine | Pre-resected PDAC | 15 |
| NCT04117087 [39] | I | KRAS peptide vaccine, nivolumab, and ipilimumab | Pre-resected PDAC | 30 |
| NCT01088789 [103] | II | Multiple cohorts and arms involving allogenic pancreatic tumor cell vaccine transfected with GM-CSF, in combination with cyclophosphamide | Pre-resected PDAC | 72 |
| NCT03592888 [104] | I | mDC3/8-KRAS vaccine | Pre-resected PDAC | 12 |
| NCT02600949 [105] | I | Multiple cohorts testing personalized vaccine + imiquimod with pembrolizumab and APX005M | Advanced PDAC or colorectal cancer | 150 |
| NCT03006302 [106] | II | Epacadostat + pembrolizumab + CY + GVAX + CRS-207 | mPDAC | 44 |
| NCT04157127 [107] | I | Autologous DC vaccine | PDAC | 43 |
| NCT02451982 [41] | I | Arm A: CY/GVAX alone Arm B: CY/GVAX + nivolumab Arm C: CY/GVAX + nivolumab + urelumab Arm D: BMS-986253 + nivolumab | Resectable adenocarcinoma of the pancreas | 76 |
| NCT03767582 [46] | I-II | Phase I: GVAX/Nivolumab/CCR2/CCR5 dual antagonist Phase II: Arm A: nivolumab/CCR2/CCR5 dual antagonist Arm B: nivolumab/GVAX/CCR2/CCR5 dual antagonist | Locally PDAC | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, C.; Alqahtani, A.; Noel, M.S. The Next Frontier in Pancreatic Cancer: Targeting the Tumor Immune Milieu and Molecular Pathways. Cancers 2022, 14, 2619. https://doi.org/10.3390/cancers14112619
Yin C, Alqahtani A, Noel MS. The Next Frontier in Pancreatic Cancer: Targeting the Tumor Immune Milieu and Molecular Pathways. Cancers. 2022; 14(11):2619. https://doi.org/10.3390/cancers14112619
Chicago/Turabian StyleYin, Chao, Ali Alqahtani, and Marcus S. Noel. 2022. "The Next Frontier in Pancreatic Cancer: Targeting the Tumor Immune Milieu and Molecular Pathways" Cancers 14, no. 11: 2619. https://doi.org/10.3390/cancers14112619