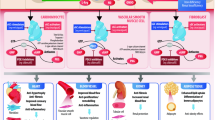
Abstract
Vericiguat (Verquvo®) is the first oral soluble guanylate cyclase (sGC) stimulator to be approved for the treatment of adults with symptomatic, chronic heart failure with reduced ejection fraction (HFrEF). In the phase III VICTORIA trial, vericiguat added to standard of care (SOC) was associated with a significantly lower risk of the primary composite endpoint of death from cardiovascular (CV) causes or first hospitalization from heart failure (HHF) than placebo added to SOC in adults with chronic HFrEF. The risk of all-cause mortality or first HHF (secondary composite endpoint) and the total number of HHF were also statistically significantly reduced by vericiguat therapy. Vericiguat showed no benefit with respect to the primary endpoint in a subgroup of patients with grossly elevated N-terminal pro-brain natriuretic peptide levels. Vericiguat was generally well tolerated; the most common treatment-related adverse event (AE) was hypotension. AEs of special interest included symptomatic hypotension and syncope, which occurred with low incidences that were similar between treatment groups. Thus, vericiguat is an effective and generally well-tolerated treatment option in patients with symptomatic, chronic HFrEF who have experienced a recent worsening event, expanding the options currently available for chronic HFrEF management.
Plain Language Summary
Approximately half of patients with heart failure develop chronic heart failure with reduced ejection fraction (HFrEF). Despite using various standard treatments that are available, some patients with symptomatic, chronic HFrEF still develop worsening heart failure. Soluble guanylate cyclase (sGC) stimulators are an emerging treatment option for heart failure management. They work by stimulating sGC activity (which is reduced in patients with heart failure), with potential benefits for myocardial and vascular function. Vericiguat (Verquvo®) is the first oral sGC stimulator to be approved for the treatment of adults with symptomatic, chronic HFrEF. When added to standard treatment(s) for chronic HFrEF, vericiguat reduced the combined risk of death from cardiovascular causes or first hospitalization for heart failure. This was primarily driven by a reduction in hospitalizations for heart failure (rather than in mortality). Vericiguat was generally well tolerated in these patients, and the incidences of adverse events of special interest such as symptomatic low blood pressure and fainting were low and similar between vericiguat and placebo recipients. Thus, vericiguat is an effective and well-tolerated treatment option in patients with symptomatic, chronic HFrEF who have experienced a recent worsening event.


Similar content being viewed by others
Change history
16 June 2022
The correct version of the video file is uploaded.
References
Roger VL. Epidemiology of heart failure: a contemporary perspective. Circ Res. 2021;128(10):1421–34.
McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726.
Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart failure with reduced ejection fraction: a review. JAMA. 2020;324(5):488–504.
McDonald M, Virani S, Chan M, et al. CCS/CHFS Heart Failure Guidelines update: defining a new pharmacologic standard of care for heart failure with reduced ejection fraction. Can J Cardiol. 2021;37(4):531–46.
Butler J, Yang M, Manzi MA, et al. Clinical course of patients with worsening heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73(8):935–44.
Zheng X, Zheng W, Xiong B, et al. The efficacy and safety of soluble guanylate cyclase stimulators in patients with heart failure: a systematic review and meta-analysis. Medicine (Baltimore). 2018;97(41):e12709.
Merck Sharp & Dohme Corp. VERQUVO® (vericiguat) tablets, for oral use: US prescribing information. 2021. https://dailymed.nlm.nih.gov. Accessed 20 Apr 2022.
Hulot J-S, Trochu J-S, Donal E, et al. Vericiguat for the treatment of heart failure: mechanism of action and pharmacological properties compared with other emerging therapeutic options. Expert Opin Pharmacother. 2021;22(14):1847–55.
European Medicines Agency. VERQUVO® (vericiguat): EU summary of product characteristics. 2021. https://www.ema.europa.eu. Accessed 20 Apr 2022.
Armstrong PW, Roessig L, Patel MJ, et al. A multicenter, randomized, double-blind, placebo-controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA trial. JACC Heart Fail. 2018;6(2):96–104.
Gheorghiade M, Marti CN, Sabbah HN, et al. Soluble guanylate cyclase: a potential therapeutic target for heart failure. Heart Fail Rev. 2013;18(2):123–34.
Gheorghiade M, Greene SJ, Butler J, et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES-REDUCED randomized trial. JAMA. 2015;314(21):2251–62.
Kramer F, Voss S, Roessig L, et al. Evaluation of high-sensitivity C-reactive protein and uric acid in vericiguat-treated patients with heart failure with reduced ejection fraction. Eur J Heart Fail. 2020;22(9):1675–83.
Ruehs H, Klein D, Frei M, et al. Population pharmacokinetics and pharmacodynamics of vericiguat in patients with heart failure and reduced ejection fraction. Clin Pharmacokinet. 2021;60(11):1407–21.
Boettcher M, Gerisch M, Lobmeyer M, et al. Metabolism and pharmacokinetic drug-drug interaction profile of vericiguat, a soluble guanylate cyclase stimulator: results from preclinical and phase I healthy volunteer studies. Clin Pharmacokinet. 2020;59(11):1407–18.
Boettcher M, Loewen S, Gerrits M, et al. Pharmacodynamic and pharmacokinetic interaction profile of vericiguat: results from three randomized phase I studies in healthy volunteers. Clin Pharmacokinet. 2021;60(3):337–51.
Boettcher M, Thomas D, Mueck W, et al. Safety, pharmacodynamic, and pharmacokinetic characterization of vericiguat: results from six phase I studies in healthy subjects. Eur J Clin Pharmacol. 2021;77(4):527–37.
Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382(20):1883–93.
European Medicines Agency. Verquvo: EPAR - public assessment report. 2021. https://www.ema.europa.eu. Accessed 20 Apr 2022.
Lam CSP, Giczewska A, Sliwa K, et al. Clinical outcomes and response to vericiguat according to index heart failure event: insights from the VICTORIA trial. JAMA Cardiol. 2021;6(6):706–12.
Voors AA, Mulder H, Reyes E, et al. Renal function and the effects of vericiguat in patients with worsening heart failure with reduced ejection fraction: insights from the VICTORIA (Vericiguat Global Study in Subjects with HFrEF) trial. Eur J Heart Fail. 2021;23(8):1313–21.
Ezekowitz JA, O’Connor CM, Troughton RW, et al. N-terminal pro-B-type natriuretic peptide and clinical outcomes: vericiguat heart failure with reduced ejection fraction study. JACC Heart Fail. 2020;8(11):931–9.
DeFilippi CR, Alemayehu W, Voors A, et al. Baseline cardiac troponin T, clinical outcomes and vericiguat treatment effect in heart failure with reduced ejection fraction study: insights from the VICTORIA trial. J Am Coll Cardiol. 2021;77(18 Suppl 1):565.
Ponikowski P, Alemayehu W, Oto A, et al. Vericiguat in patients with atrial fibrillation and heart failure with reduced ejection fraction: insights from the VICTORIA trial. Eur J Heart Fail. 2021;23(8):1300–12.
Ezekowitz J, Zheng Y, Lund L, et al. Changes in natriuretic peptide and effects of vericiguat in worsening heart failure with reduced ejection fraction: insights from VICTORIA (VerICiguaT Global Study in Subjects with HFrEF) [abstract]. Eur J Heart Fail. 2021;23(Suppl S2):2–322.
Lam CSP, Mulder H, Lopatin Y, et al. Blood pressure response and safety outcomes with vericiguat in the VICTORIA trial [abstract]. Eur J Heart Fail. 2021;23(Suppl S2):2–322.
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79(17):e263–421.
Butler J, Djatche LM, Lautsch D, et al. Representativeness of the VICTORIA trial population in clinical practice: analysis of the PINNACLE registry. J Card Fail. 2021;27(12):1374–81.
Mentz RJ, Mulder H, Mosterd A, et al. Clinical outcome predictions for the Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction (VICTORIA) trial. J Card Fail. 2021;27(9):949–56.
Aimo A, Pateras K, Stamatelopoulos K, et al. Relative efficacy of sacubitril-valsartan, vericiguat, and SGLT2 inhibitors in heart failure with reduced ejection fraction: a systematic review and network meta-analysis. Cardiovasc Drugs Ther. 2020;35(5):1067–76.
Tromp J, Ouwerkerk W, van Veldhuisen D, et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10(2):73–84.
Scalvini S, Bernocchi P, Villa S, et al. Treatment prescription, adherence, and persistence after the first hospitalization for heart failure: a population-based retrospective study on 100785 patients. Int J Cardiol. 2021;330:106–11.
Savarese G, Stolfo D, Sinagra G, et al. Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol. 2022;19(2):100–16.
Alsumali A, Djatche LM, Briggs A, et al. Cost effectiveness of vericiguat for the treatment of chronic heart failure with reduced ejection fraction following a worsening heart failure event from a US Medicare perspective. Pharmacoeconomics. 2021;39(11):1343–54.
Alsumali A, Lautsch D, Liu R, et al. Budget impact analysis of vericiguat for the treatment of chronic heart failure with reduced ejection fraction following a worsening event. Adv Ther. 2021;38(5):2631–43.
Acknowledgments
During the peer review process, the manufacturer of vericiguat was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Funding
The preparation of this review was not supported by any external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authorship and Conflict of interest
Connie Kang and Yvette N. Lamb are salaried employees of Adis International Ltd/Springer Nature, and declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: D. Abramov, Department of Medicine, Loma Linda University Medical School, Loma Linda, CA, USA; W. S. Aronow, Department of Cardiology, Westchester Medical Center & New York Medical College, Valhalla, NY, USA; J. G. F. Cleland, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow and National Heart & Lung Institute, Imperial College London, London, UK.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kang, C., Lamb, Y.N. Vericiguat: A Review in Chronic Heart Failure with Reduced Ejection Fraction. Am J Cardiovasc Drugs 22, 451–459 (2022). https://doi.org/10.1007/s40256-022-00538-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-022-00538-5