Abstract
Background
Primary tumor site and genomic status are utilized for regimen selection in metastatic colorectal cancer; however, the impact on clinical practice is not well known.
Objective
We aimed to clarify the impact of primary tumor site and genomic status on clinical practice in metastatic colorectal cancer.
Methods
The relationship between primary tumor site, genomic alterations, and clinical outcomes was evaluated in patients with untreated metastatic colorectal cancer using real-world data of a prospective observational study, SCRUM-Japan GI-SCREEN with clinical and genomic data set in 1011 patients enrolled from February 2015 to March 2017.
Results
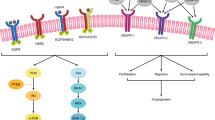
Five hundred and sixty-one patients were eligible for this study. Patients with right-sided tumors had a significantly worse survival, left-sided tumors with wild-type RAS had favorable outcomes when treated with anti-epidermal growth factor receptor monoclonal antibodies, and cecum tumors had poor prognosis when treated with bevacizumab. The rate of gene alterations varied considerably depending on the primary site. In addition, gene alterations of KRAS, BRAF, SMAD4, or TP53 had individually different contributions to survival from site to site. KRAS, BRAF, PTEN, or SMAD4 mutations were associated with efficacy of bevacizumab or anti-epidermal growth factor receptor monoclonal antibodies.
Conclusions
Primary tumor site is a clinically useful biomarker to predict survival in patients with metastatic colorectal cancer treated with first-line chemotherapy. Moreover, the prognostic or predictive value of several gene alterations by primary tumor site should be considered in clinical practice.





Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. https://doi.org/10.1002/ijc.29210.
Mizukami T, Izawa N, Nakajima TE, Sunakawa Y. Targeting EGFR and RAS/RAF signaling in the treatment of metastatic colorectal cancer: from current treatment strategies to future perspectives. Drugs. 2019;79(6):633–45. https://doi.org/10.1007/s40265-019-01113-0.
Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32(21):2240–7. https://doi.org/10.1200/JCO.2013.53.2473.
Elez E, Argiles G, Tabernero J. First-line treatment of metastatic colorectal cancer: interpreting FIRE-3, PEAK, and CALGB/SWOG 80405. Curr Treat Options Oncol. 2015;16(11):52. https://doi.org/10.1007/s11864-015-0369-x.
Rivera F, Karthaus M, Fasola G, Canon J-L, Hecht JR, Tian Y, et al. Extended RAS analysis and subsequent anti-EGFR and anti-VEGF treatment (tx) in PEAK: a first-line phase 2 study of FOLFOX6 + panitumumab (pmab) or bevacizumab (bev) in metastatic colorectal cancer (mCRC). J Clin Oncol. 2014;32(15_Suppl.):3629.
Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK. Is right-sided colon cancer different to left-sided colorectal cancer? A systematic review. Eur J Surg Oncol. 2015;41(3):300–8. https://doi.org/10.1016/j.ejso.2014.11.001.
Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. 2017;3(2):211–9. https://doi.org/10.1001/jamaoncol.2016.4227.
Venook AP, Lenz J-J, Kabbarah O, Qu X, Niedzwiecki D, Zemla T, et al. Primary (1°) tumor location as an independent prognostic marker from molecular features for overall survival (OS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2017;35(15_Suppl.):3503.
Loupakis F, Yang D, Yau L, Feng S, Cremolini C, Zhang W, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015. 107(3). https://doi.org/10.1093/jnci/dju427.
Sunakawa Y, Ichikawa W, Tsuji A, Denda T, Segawa Y, Negoro Y, et al. Prognostic impact of primary tumor location on clinical outcomes of metastatic colorectal cancer treated with cetuximab plus oxaliplatin-based chemotherapy: a subgroup analysis of the JACCRO CC-05/06 trials. Clin Colorectal Cancer. 2017;16(3):e171–80. https://doi.org/10.1016/j.clcc.2016.09.010.
Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713–29. https://doi.org/10.1093/annonc/mdx175.
Khattak MA, Martin H, Davidson A, Phillips M. Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: a meta-analysis of randomized clinical trials. Clin Colorectal Cancer. 2015;14(2):81–90. https://doi.org/10.1016/j.clcc.2014.12.011.
Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87–98. https://doi.org/10.1016/j.ejca.2016.10.007.
National Comprehensive Cancer Network. Colon cancer. Version 1. 2022. Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed May 2, 2022.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–422. https://doi.org/10.1093/annonc/mdw235.
Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS. SSO and TOS Ann Oncol. 2018;29(1):44–70. https://doi.org/10.1093/annonc/mdx738.
Loree JM, Pereira AAL, Lam M, Willauer AN, Raghav K, Dasari A, et al. Classifying colorectal cancer by tumor location rather than sidedness highlights a continuum in mutation profiles and consensus molecular subtypes. Clin Cancer Res. 2018;24(5):1062–72. https://doi.org/10.1158/1078-0432.CCR-17-2484.
Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86: 102017. https://doi.org/10.1016/j.ctrv.2020.102017.
Price TJ, Beeke C, Ullah S, Padbury R, Maddern G, Roder D, et al. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer. 2015;121(6):830–5. https://doi.org/10.1002/cncr.29129.
Wang ZH, Gao QY, Fang JY. Loss of PTEN expression as a predictor of resistance to anti-EGFR monoclonal therapy in metastatic colorectal cancer: evidence from retrospective studies. Cancer Chemother Pharmacol. 2012;69(6):1647–55. https://doi.org/10.1007/s00280-012-1886-y.
Therkildsen C, Bergmann TK, Henrichsen-Schnack T, Ladelund S, Nilbert M. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: a systematic review and meta-analysis. Acta Oncol. 2014;53(7):852–64. https://doi.org/10.3109/0284186X.2014.895036.
Mei Z, Shao YW, Lin P, Cai X, Wang B, Ding Y, et al. SMAD4 and NF1 mutations as potential biomarkers for poor prognosis to cetuximab-based therapy in Chinese metastatic colorectal cancer patients. BMC Cancer. 2018;18(1):479. https://doi.org/10.1186/s12885-018-4298-5.
Loupakis F, Cremolini C, Salvatore L, Masi G, Sensi E, Schirripa M, et al. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur J Cancer. 2014;50(1):57–63. https://doi.org/10.1016/j.ejca.2013.08.024.
Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–15. https://doi.org/10.1016/S1470-2045(15)00122-9.
Salvatore L, Calegari MA, Loupakis F, Fassan M, Di Stefano B, Bensi M, et al. PTEN in colorectal cancer: shedding light on its role as predictor and target. Cancers (Basel). 2019. 11(11):1765. https://doi.org/10.3390/cancers11111765.
Xie MZ, Li JL, Cai ZM, Li KZ, Hu BL. Impact of primary colorectal cancer location on the KRAS status and its prognostic value. BMC Gastroenterol. 2019;19(1):46. https://doi.org/10.1186/s12876-019-0965-5.
Cremolini C, Antoniotti C, Lonardi S, Bergamo F, Cortesi E, Tomasello G, et al. Primary tumor sidedness and benefit from FOLFOXIRI plus bevacizumab as initial therapy for metastatic colorectal cancer: retrospective analysis of the TRIBE trial by GONO. Ann Oncol. 2018;29(7):1528–34. https://doi.org/10.1093/annonc/mdy140.
Sinicrope FA, Shi Q, Allegra CJ, Smyrk TC, Thibodeau SN, Goldberg RM, et al. Association of DNA mismatch repair and mutations in BRAF and KRAS with survival after recurrence in stage III colon cancers: a secondary analysis of 2 randomized clinical trials. JAMA Oncol. 2017;3(4):472–80. https://doi.org/10.1001/jamaoncol.2016.5469.
Taniguchi H, Uehara K, Nakayama G, Nakayama H, Aiba T, Hattori N, et al. Tumor location is associated with the prevalence of Braf and Pik3ca mutations in patients with wild-type Ras colorectal cancer: a prospective multi-center cohort study in Japan. Transl Oncol. 2020;13(7): 100786. https://doi.org/10.1016/j.tranon.2020.100786.
Network CGA. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7. https://doi.org/10.1038/nature11252.
Samowitz WS, Curtin K, Schaffer D, Robertson M, Leppert M, Slattery ML. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol Biomark Prev. 2000;9(11):1193–7.
Modest DP, Ricard I, Heinemann V, Hegewisch-Becker S, Schmiegel W, Porschen R, et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. 2016;27(9):1746–53. https://doi.org/10.1093/annonc/mdw261.
Phipps AI, Limburg PJ, Baron JA, Burnett-Hartman AN, Weisenberger DJ, Laird PW, et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015;148(1):77-87.e2. https://doi.org/10.1053/j.gastro.2014.09.038.
Venderbosch S, Nagtegaal ID, Maughan TS, Smith CG, Cheadle JP, Fisher D, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20(20):5322–30. https://doi.org/10.1158/1078-0432.CCR-14-0332.
Gonsalves WI, Mahoney MR, Sargent DJ, Nelson GD, Alberts SR, Sinicrope FA, et al. Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147. J Natl Cancer Inst. 2014. 106(7). https://doi.org/10.1093/jnci/dju106.
Nakamura Y, Fujisawa T, Taniguchi H, Bando H, Okamoto W, Tsuchihara K, et al. SCRUM-Japan GI-SCREEN and MONSTAR-SCREEN: path to the realization of biomarker-guided precision oncology in advanced solid tumors. Cancer Sci. 2021;112(11):4425–32. https://doi.org/10.1111/cas.15132.
Acknowledgements
The authors thank all patients and their families who participated in this study; all SCRUM-Japan GI-SCREEN investigators and site personnel; and Ms. Yumiko Yamaguchi and Ms. Mayumi Ushitani (Clinical Research Data Center, St. Marianna University School of Medicine) for data center support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by SCRUM-Japan Funds.
Conflicts of interest/competing interests
Takuro Mizukami reports grants and personal fees from Taiho Pharmaceutical, Eli Lilly Japan, and Ono Pharmaceutical as well as personal fees from Otsuka Pharmaceutical Factory, Asahi Kasei Pharmaceutical, Merck Biopharma, Sanofi, and Takeda Pharmaceutical. Yu Sunakawa reports grants and personal fees from Taiho Pharmaceutical, Chugai Pharma, Takeda, Eli Lilly Japan, and Sanofi; personal fees from Bayer Yakuhin, Yakult Honsha, Bristol-Myers Squibb Japan, Merck Biopharma, Nippon Kayaku, Kyowa Hakko Kirin, Ono Pharmaceutical, MSD, and Daiichi Sankyo; and grants from Otsuka. Satoshi Yuki reports personal fees from Chugai Pharmaceutical Co., Ltd., Eli Lilly K.K., Takeda Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd, Bristol-Myers Squibb Co., Ltd., Taiho Pharmaceutical Co., Ltd., MSD K.K., Ono Pharmaceutical Co., Ltd., Medical & Biological Laboratories Co., Ltd., Yakult Honsha Co., Ltd., Merck Biopharma Co., Ltd., and Sanofi K.K. Yoshinori Kagawa reports personal fees from Bayer Co., Ltd., Chugai Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Sanofi Co., Ltd., Eli Lilly Japan Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Merck Co., Ltd. Atsushi Takashima reports personal fees from Lilly, Taiho Pharmaceutical, Chugai Pharma, and Merck Serono; grants and personal fees from Ono Pharmaceutical and Takeda; and grants from Merck Sharp & Dohme, Eisai, Eisai, Bayer Yakuhin, and Bristol-Myers Squibb. Hiroshi Hara reports grants from AstraZeneca, Eisai, Elevar Therapeutics, Astellas, Beigene, Incyte, and Pfizer; grants and personal fees from Daiichi Sankyo, Dainippon Sumitomo Pharma, Merck Biopharma, MSD, Taiho, Chugai, Boehringer-Ingelheim, Ono Pharmaceutical, and BMS; and personal fees from Lilly, Yakult Honsha, Sanofi, Takeda, and Kyowa Hakko Kirin. Tadamichi Denda reports personal fees from Sawai Pharmaceutical Co and Sysmex as well as grants from MSD and Ono Pharmaceutical. Eiji Oki reports other from Chugai, Merck Biopharm, Eli Lilly, Takeda Pharm, Taiho Pharm, Bayer, and Takeda Pharm. Okamoto reports personal fees from Chugai Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, Yakult Honsha, and Thermo Fisher Scientific as well as grants from Janssen Pharmaceutical. Takayuki Yoshino reports grants from Taiho Pharmaceutical, Sumitomo Dainippon Pharma, Ono Pharmaceutical, Chugai Pharmaceutical, Amgen, PAREXEL International, MSD, Daiichi Sankyo, and Sanofi. Takako Eguchi Nakajima reports grants from the National Cancer Research and Development Fund (26-A-4, 29-A-3), grants from Grant-in-Aid for Clinical Cancer Research (H26-144) from the Ministry of Health, Labour and Welfare of Japan, and grants from Japan Agency for Medical Research and Development (Grant numbers JP16ck0106139 and JP18ck0106351), during the conduct of the study; grants and personal fees from Sumitomo Dainippon Pharma Co., personal fees from Boehringer Ingelheim, Bristol-Myers Squibb, Novartis Japan, Bayer Yakuhin, Pfizer Japan Inc., Yakult Honsha Co., Nipro Co., Celltrion Healthcare Japan, Teijin Pharma, and Sawai Pharmaceutical Co., and grants and personal fees from Ono Pharmaceutical Co., Taiho Pharmaceutical Co., Amgen, Takeda Pharmaceutical Co., Chugai Pharmaceutical Co., Sanofi K.K., Nippon Kayaku Co., MSD K.K., Eli Lilly Japan K.K., Daiichi Sankyo Co., and Merck Serono Co., and grants from Eisai Co, outside the submitted work. Masaki Takahashi, Kyoko Kato, Youhei Yamamoto, and Manabu Shiozawa declare that they have no conflicts of interest that might be relevant to the contents of this article.
Ethics approval
This study is conducted following the Declaration of Helsinki and Ethical Guidelines for Medical and Health Research Involving Human Subjects and has been approved by the Institutional Review Boards and the Research Ethics Committee of St. Marianna University School of Medicine and National Cancer Center East.
Consent to participate
All participants provided written informed consent for participation.
Consent for publication
This article does not disclose any personally identifiable information of any of the participants. Hence, consent is not applicable.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
TM: conceptualization, methodology, provision, data curation, writing of the original draft, visualization; MT: software, validation, formal analysis, data curation; YS: investigation, methodology, writing of the original draft; SY: investigation, writing, reviewing, and editing; YK: investigation, writing, reviewing, and editing; AT: investigation, writing, reviewing, and editing; KK: investigation, writing, reviewing, and editing; HH: investigation, writing, reviewing, and editing; TD: investigation, writing, reviewing, and editing; YY: investigation, writing, reviewing, and editing; MS: investigation, writing, reviewing, and editing; EO: investigation, writing, reviewing, and editing; WO: investigation, writing, reviewing, and editing; TY: investigation, writing, reviewing, and editing, supervision, funding acquisition; TEN: conceptualization, methodology, writing of the original draft, supervision, project administration, funding acquisition.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mizukami, T., Takahashi, M., Sunakawa, Y. et al. Genomic Landscape of Primary Tumor Site and Clinical Outcome for Patients with Metastatic Colorectal Cancer Receiving Standard-of-Care Chemotherapy. Targ Oncol 17, 343–353 (2022). https://doi.org/10.1007/s11523-022-00880-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00880-3