Abstract
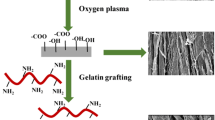
Some disadvantages of commercial dressings have mobilized research groups to clinically improve these materials considering their cost–benefit ratio. Tissue engineering has emerged as a field that uses biomaterials in the form of scaffolds mimicking human morphofunctionality and their combination with cells in order to repair or replace damaged tissues. There is still a need for customization and characterization of the material to ensure its safety. Therefore, this study aimed to perform further characterizations of the gelatin (Gel)–coated poly(ε-caprolactone) (PCL) matrix produced in previous studies by rotary jet spinning. PCL fibers produced by rotary jet spinning were submersed in a Gel solution, inspired by the dip-coating method, followed by crosslinking with glutaraldehyde. The material was then submitted to physicochemical and biological characterization. The thermal properties were confirmed by differential scanning calorimetry. Significant differences were observed in the fiber thickness and swelling of PCL/Gel compared to PCL, while fiber spacing was greater for PCL. The crosslinked Gel appeared to increase the tensile strength of PCL, and the thermal stability of PCL/Gel was higher than that of Gel alone. In addition to the absence of cytotoxicity of the materials, the cytochemical data suggest higher cell activity on the PCL/Gel scaffold. Scanning electron microscopy reinforces this observation, indicating promising applications of PCL/Gel scaffolds in skin tissue engineering.











Similar content being viewed by others
References
McLaughlin ES, Paterson AO (2012). Burns: prevention, causes and treatment. Surgery—procedures, complications, and results: human anatomy and physiology. Nova Science Publishers, New York. https://www.biosanas.com.br/uploads/outros/artigos_cientificos/58/09eb10009ec9bdd82a6f555f0042561c.pdf
Wang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R, Lajevardi SS, Li Z, Maitz PKM (2018) Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev 123:3–17. https://doi.org/10.1016/j.addr.2017.09.018
Brasil. Ministério da Saúde (2009) Portaria nº 2.600, de 21 de outubro de 2009. Aprova o Regulamento Técnico do Sistema Nacional de Transplantes. Diário Oficial da União. https://bvsms.saude.gov.br/bvs/saudelegis/gm/2009/prt2600_21_10_2009.html
Lima EM, Moraes-Filho MO, Rocha MBS, Silva-Júnior FR, Leontsinis CMP, Nascimento MFA (2019) Elaboração, desenvolvimento e instalação do primeiro banco de pele animal no Brasil para o tratamento de queimaduras e feridas. Rev Bras Cir Plást 34(3):349–354
Giorno LP, Rodrigues LR, Santos AR Jr (2018) Métodos avançados para tratamento de queimaduras: uma revisão. Rev Bras Queimadura 17(1):60–65
World Health Organization (2018) WHO. https://www.who.int/news-room/fact-sheets/detail/burns
Langer R, Vacanti JP (1993) Tissue engineering. Science 260(5110):920–926. https://doi.org/10.1126/science.8493529
Hench LL (1993) Biomaterials: a forecast for the future. Biomaterials 19(16):1419–1423. https://doi.org/10.1016/S0142-9612(98)00133-1
Halim AS, Khoo TL, Mohd Yussof SJ (2010) Biologic and synthetic skin substitutes: an overview. Indian J Plast Surg 43(Suppl):S23-28
Jones V, Grey JE, Harding KG (2006) ABC of wound healing: wound dressings. BMJ 332(7544):777–780. https://doi.org/10.1136/bmj.332.7546.900
Giorno LP, Rodrigues LR, Santos AR Jr (2019) Graft technologies: an overview. Stem Cell Res Ther 4(1):135–141
Jiang S, Li SC, Huang C, Chan BP, Du Y (2018) Physical properties of implanted porous bioscaffolds regulate skin repair: focusing on mechanical and structural features. Adv Healthc Mater 7(6):e1700894. https://doi.org/10.1002/adhm.201700894
Batalha E (2017) Uso de animais em pesquisa abrange desafios éticos e compromisso com novas tecnologias. Revista Radis. https://portal.fiocruz.br/noticia/uso-de-animais-em-pesquisa-abrange-desafios-eticos-e-compromisso-com-novas-tecnologias
Liu D, Nikoo M, Boran G, Zhou P, Regenstein JM (2015) Collagen and gelatin. Annu Rev Food Sci Technol 6:527–557. https://doi.org/10.1146/annurev-food-031414-111800
Farris S, Song J, Huang Q (2010) Alternative reaction mechanism for the crosslinking of gelatin with glutaraldehyde. J Agric Food Chem 58(2):998–1003. https://doi.org/10.1021/jf9031603
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37(5):798–802. https://doi.org/10.2144/04375RV01
Bigi A, Cojazzi G, Panzavolta S, Rubini K, Roveri N (2001) Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 22(8):763–768. https://doi.org/10.1016/S0142-9612(00)00236-2
Woodruff MA, Hutmacher DW (2010) The return of a forgotten polymer—polycaprolactone in the 21st century. Prog Polym Sci 35(10):1217–1256. https://doi.org/10.1016/j.progpolymsci.2010.04.002
Loordhuswamy AM, Krishnaswamy VR, Korrapati PS, Thinakaran S, Rengaswami GD (2014) Fabrication of highly aligned fibrous scaffolds for tissue regeneration by centrifugal spinning technology. Mater Sci Eng C Mater Biol Appl 42:799–807. https://doi.org/10.1016/j.msec.2014.06.011
Sharif S, Ai J, Azami M, Verdi J, Atlasi MA, Shirian S, Samadikuchaksaraei A (2018) Collagen-coated nano-electrospun PCL seeded with human endometrial stem cells for skin tissue engineering applications. J Biomed Mater Res B Appl Biomater 106(4):1578–1586. https://doi.org/10.1002/jbm.b.33966
Tang X, Yan X (2016) Dip-coating for fibrous materials: mechanism, methods and applications. J Sol-Gel Sci Technol 81:378–404. https://doi.org/10.1007/s10971-016-4197-7
Raut HK, Ganesh VA, Nair AS, Ramakrishna S (2011) Anti-reflective coatings: a critical, in-depth review. Energy Environ Sci 4(10):3779–3804. https://doi.org/10.1039/C1EE01297E
Schabikowski M, Tomaszewska J, Kata D, Graule T (2015) Rotary jet-spinning of hematite fibers. Text Res J 85(3):316–324. https://doi.org/10.1177/0040517514542969
Badrossamay MR, McIlwee HA, Goss JA, Parker KK (2010) Nanofiber assembly by rotary jet-spinning. Nano Lett 10(6):2257–2261. https://doi.org/10.1021/nl101355x
Rodrigues ICP, Woigt LF, Pereira KD, Luchessi AD, Lopes ESN, Webster TJ, Gabriel LP (2020) Low-cost hybrid scaffolds based on polyurethane and gelatin. J Mater Res Technol 9(4):7777–7785. https://doi.org/10.1016/j.jmrt.2020.04.049
Giorno LP, Rodrigues LR, Santos Jr AR (2020) Fibrous PCL scaffolds as tissue substitutes. Int J Adv Med Biotechnol 3(1):40–45. https://doi.org/10.25061/ijamb.v3i1.64
ISO 10993-5:2009(en) Biological evaluation of medical devices—part 5: tests for in vitro cytotoxicity. https://www.iso.org/obp/ui/#iso:std:iso:10993:-5:ed-3:v1:en
BRASIL. Resolução RDC nº 185, de 22 de outubro de 2001. Aprova o Regulamento Técnico que consta no anexo desta Resolução, que trata do “registro, alteração, revalidação e cancelamento do registro de produtos médicos”. ANVISA - Agência Nacional de Vigilância Sanitária. https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2001/rdc0185_22_10_2001.pdf
International Standard (2009) ISO 10993-5 (E): biological evaluation of medical devices. Part 5: tests for cytotoxicity: in vitro methods. https://www.iso.org/standard/36406.html
Levinson H, Lorden ER, Brown DA (2019) U.S. patent application no. 16/318,855
Roa JP, Mano V, Faustino PB, Felix EB, Silva MESR, Souza Filho JD (2010) Síntese e caracterização do copolímero Poli (3-hidroxibutirato-co-ε-caprolactona) a partir de Poli (3-hidroxibutirato e Poli (ε-caprolactona). Polímeros 20(3):221–226. https://doi.org/10.1590/S0104-14282010005000038
De Kesel C, Lefevre C, Nagy JB, David C (1999) Blends of polycaprolactone with polyvinylalcohol: a DSC, optical microscopy and solid state NMR study. Polymer 40(8):1969–1978. https://doi.org/10.1016/S0032-3861(98)00253-5
ASTM D638M-96. Standard test method for tensile properties of plastics (metric). https://standards.globalspec.com/std/3802749/astm-d638m-96
ASTM Standard practice for direct contact cell culture evaluation of materials for medical devices. Annu B ASTM Stand. 2011;07 (Reapproved 2012):5–8. https://standards.globalspec.com/std/14302429/astm-f813-20
Mello MLS (1997) Cytochemistry of DNA, RNA and nuclear proteins. Braz J Genet 20:257–264
Slade L, Levine H, Finley JW (1989) Protein-water interactions: water as a plasticizer of gluten and other polymers. In: Phillips RD, Finley JW (eds) Protein quality and the effects of processing. Marcel Dekker, Inc., New York, pp 9–124
Mukherjee I, Rosolen M (2013) Thermal transitions of gelatin evaluated using DSC sample pans of various seal integrities. J Therm Anal Calorim 114(3):1161–1166. https://doi.org/10.1007/s10973-013-3166-4
Rogalski JJ, Bastiaansen CW, Peijs T (2017) Rotary jet spinning review—a potential high yield future for polymer nanofibers. Nanocomposites 3(4):97–121. https://doi.org/10.1080/20550324.2017.1393919
Moraes Segundo JDP, Moraes MOS, Brito WR, D’Ávila MA (2020) Incorporation of molecularly imprinted polymer nanoparticles in electrospun polycaprolactone fibers. Mater Lett 275:128088. https://doi.org/10.1016/j.matlet.2020.128088
Nagiah N, Johnson R, Anderson R, Elliott W, Tan W (2015) Highly compliant vascular grafts with gelatin-sheathed coaxially structured nanofibers. Langmuir 31(47):12993–13002. https://doi.org/10.1021/acs.langmuir.5b03177
Prado-Prone G, Bazzar M, Focarete ML, Garcia-Macedo J, Perez-Orive J, Ibarra C, Velasquillo C, Silva-Bermudez P (2020) Single-step, acid-based fabrication of homogeneous gelatin–polycaprolactone fibrillar scaffolds intended for skin tissue engineering. Biomed Mater 15(3):035001. https://doi.org/10.1088/1748-605X/ab673b
Pereira RF, Barrias CC, Granja PL, Bartolo PJ (2013) Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine 8(4):603–621. https://doi.org/10.2217/nnm.13.50
Ghasemkhah F, Latifi M, Hadjizadeh A, Shokrgozar MA (2019) Potential coreshell designed scaffolds with a gelatin-based shell in achieving controllable release rates of proteins for tissue engineering approaches. J Biomed Mater Res A 107(7):1393–1405. https://doi.org/10.1002/jbm.a.36653
Badrossamay MR, Balachandran K, Capulli AK, Golecki HM, Agarwal A, Goss JA, Kim H, Shin K, Parker KK (2014) Engineering hybrid polymer-protein superaligned nanofibers via rotary jet spinning. Biomaterials 35(10):3188–3197. https://doi.org/10.1016/j.biomaterials.2013.12.072
Cardoso GB, Machado-Silva AB, Sabino M, Santos AR Jr, Zavaglia CA (2014) Novel hybrid membrane of chitosan/poly (ε-caprolactone) for tissue engineering. Biomatter 4:e29508. https://doi.org/10.4161/biom.29508
Kolbuk D, Sajkiewicz P, Denis P, Choinska E (2013) Investigations of polycaprolactone/gelatin blends in terms of their miscibility. Bull Polish Acad Sci Tech Sci 61(3):629–632. https://doi.org/10.2478/BPASTS-2013-0066
Rahman MS, Al-Saidi G, Guizani N, Abdullah A (2010) Development of state diagram of bovine gelatin by measuring thermal characteristics using differential scanning calorimetry (DSC) and cooling curve method. Thermochim Acta 509(1–2):111–119. https://doi.org/10.1016/j.tca.2010.06.011
Brasil. Ministério da Saúde. ANVISA. Resolução Nº 50/02. Dispõe sobre o Regulamento Técnico para planejamento, programação, elaboração e avaliação de projetos físicos de estabelecimentos assistenciais de saúde. https://sbim.org.br/images/legislacao/rdc-2002-50.pdf
Krasowska K, Heimowska A, Morawska M (2016) Environmental degradability of polycaprolactone under natural conditions. E3S Web Conf 10:00048. https://doi.org/10.1051/e3sconf/20161000048
Ke R, Yi W, Tao S, Wen Y, Hongyu Z (2017) Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater Sci Eng C Mater Biol Appl 78:324–332. https://doi.org/10.1016/j.msec.2017.04.084
Zadehnajar P, Karbasi S, Akbari B, Ghasemi L (2020) Incorporation of multi-walled carbon nanotubes into electrospun PCL/gelatin scaffold: the influence on the physical, chemical and thermal properties and cell response for tissue engineering. Mater Technol 35(1):39–49. https://doi.org/10.1080/10667857.2019.1651539
Zhang Y, Ouyang H, Lim CT, Ramakrishna S, Huang ZM (2005) Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res B Appl Biomater 72(1):156–165. https://doi.org/10.1002/jbm.b.30128
Croisier F, Duwez AS, Jérôme C, Léonard AF, van der Werf KO, Dijkstra PJ, Bennink ML (2012) Mechanical testing of electrospun PCL fibers. Acta Biomater 8(1):218–224. https://doi.org/10.1016/j.actbio.2011.08.015
Fu L, Xie J, Carlson MA, Reilly DA (2017) Three-dimensional nanofiber scaffolds with arrayed holes for engineering skin tissue constructs. MRS Commun 7(3):361–366. https://doi.org/10.1557/mrc.2017.49
Kishan AP, Nezarati RM, Radzicki CM, Renfro AL, Robinson JL, Whitely ME, Cosgriff-Hernandez EM (2015) In situ crosslinking of electrospun gelatin for improved fiber morphology retention and tunable degradation. J Mater Chem B 3(40):7930–7938. https://doi.org/10.1039/C5TB00937E
Humbert P, Ferial F, Agache P, Maibach HI (2004) Agache’s measuring the skin—noninvasive investigations, physiology, normal constant, 2nd edn. Springer, Berlin. https://doi.org/10.1007/978-3-319-32383-1
Dye JF, Sharma V, Garcia-Gareta E, Blackwood KA (2019) U.S. patent no. 10,357,592. U.S. Patent and Trademark Office, Washington, DC
Jiang YC, Jiang L, Huang A, Wang XF, Li Q, Turng LS (2017) Electrospun polycaprolactone/gelatin composites with enhanced cell-matrix interactions as blood vessel endothelial layer scaffolds. Mater Sci Eng C Mater Biol Appl 71:901–908. https://doi.org/10.1016/j.msec.2016.10.083
Pereira IH, Ayres E, Averous L, Schlatter G, Hebraud A, de Paula AC, Viana PH, Goes AM, Oréfice RL (2014) Differentiation of human adipose-derived stem cells seeded on mineralized electrospun co-axial poly(ε-caprolactone) (PCL)/gelatin nanofibers. J Mater Sci Mater Med 25(4):1137–1148. https://doi.org/10.1007/s10856-013-5133-9
ASTM F1635-16 (2016) Standard test method for in vitro degradation testing of hydrolytically degradable polymer resins and fabricated forms for surgical implants. ASTM International, West Conshohocken. https://standards.globalspec.com/std/10072019/ASTM%20F1635
Kuijer R, Jansen EJP, Emans PJ, Bulstra SK, Riesle J, Pieper J, Grainger DW, Busscher HJ (2007) Assessing infection risk in implanted tissue-engineered devices. Biomaterials 28(34):5148–5154. https://doi.org/10.1016/j.biomaterials.2007.06.003
Zhong SP, Zhang YZ, Lim CT (2010) Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2(5):510–525. https://doi.org/10.1002/wnan.100
Gong M, Huang C, Huang Y, Li G, Chi C, Ye J, Xie W, Shi R, Zhang L (2019) Core-sheath micro/nano fiber membrane with antibacterial and osteogenic dual functions as biomimetic artificial periosteum for bone regeneration applications. Nanomedicine 17:124–136. https://doi.org/10.1016/j.nano.2019.01.002
Bacakova L, Filova E, Parizek M, Ruml T, Svorcik V (2011) Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol Adv 29(6):739–767. https://doi.org/10.1016/j.biotechadv.2011.06.004
Khang A, Ravishankar P, Krishnaswamy A, Anderson PK, Cone SG, Liu Z, Qian X, Balachandran K (2017) Engineering anisotropic biphasic Janus-type polymer nanofiber scaffold networks via centrifugal jet spinning. J Biomed Mater Res B Appl Biomater 105(8):2455–2464. https://doi.org/10.1002/jbm.b.33791
Vida TA, Motta AC, Santos AR Jr, Cardoso GBC, Brito CC, Zavaglia CAC (2017) Fibrous PCL/PLLA scaffolds obtained by rotary jet spinning and electrospinning. Mater Res 20:910–916. https://doi.org/10.1590/1980-5373-MR-2016-0969
Baptista-Perianes A, Malmonge SM, Simbara MMO, Santos AR Jr (2019) In vitro evaluation of PHBV/PCL blends for bone tissue engineering. Mater Res 22(6):e20190338. https://doi.org/10.1590/1980-5373-MR-2019-0338
Babaloo H, Ebrahimi-Barough S, Derakhshan MA, Yazdankhah M, Lotfibakhshaiesh N, Soleimani M, Joghataei MT, Ai J (2019) PCL/gelatin nanofibrous scaffolds with human endometrial stem cells/Schwann cells facilitate axon regeneration in spinal cord injury. J Cell Physiol 234(7):11060–11069. https://doi.org/10.1002/jcp.27936
Hu Y, Feng B, Zhang W, Yan C, Yao Q, Shao C, Yu F, Li F, Fu Y (2019) Electrospun gelatin/PCL and collagen/PCL scaffolds for modulating responses of bone marrow endothelial progenitor cells. Exp Ther Med 17(5):3717–3726. https://doi.org/10.3892/etm.2019.7387
Altankov G, Brodvarova I, Rashkov I (1991) Synthesis of protein-coated gelatin microspheres and their use as microcarriers for cell culture. Part I. Derivatization with native collagen. J Biomater Sci Polym Ed 2(2):81–89. https://doi.org/10.1163/156856291X00089
Sando IC, Chung KC (2017) The use of dermal skin substitutes for the treatment of the burned hand. Hand Clin 33(2):269–276. https://doi.org/10.1016/j.hcl.2016.12.008
Acknowledgements
The authors would like to thank the Multiuser Experimental Centers (CEM) of the Federal University of ABC (UFABC), Santo André Campus and São Bernardo do Campo Campus.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giorno, L.P., Rodrigues, L.R. & Santos, A.R. Characterization and in vitro analysis of a poly(ε-caprolactone)–gelatin matrix produced by rotary jet spinning and applied as a skin dressing. Polym. Bull. 79, 9131–9158 (2022). https://doi.org/10.1007/s00289-022-04228-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04228-9