Abstract
Evaluating the dermal absorption of sunscreen UV filters requires the development of a bio-predictable in vitro permeation test (IVPT). This work describes the comparison of two IVPT methods and rank order correlations of in vitro absorption (skin permeation and retention) with the in vivo absorption (AUC and skin retention) of sunscreens. The IVPT was compared regarding the following elements: (1) application of a single finite dose vs. an infinite dose and (2) the use of heat-separated human epidermis vs. dermatomed skin models. The IVPT was used to evaluate dermal absorption of six UV filters (avobenzone, homosalate, octinoxate, octisalate, octocrylene, and oxybenzone) in commercial sunscreens. Both the in vivo and in vitro permeation studies demonstrated that all UV filters were absorbed following a single-dose application. Sunscreens were rank ordered by the amount of the UV filters absorbed. Data obtained from the IVPT method using a single finite dose and heat-separated human epidermis was found to correlate with the clinical data. Rank orders of the cumulative in vitro skin permeation and the in vivo AUC were found comparable for oxybenzone, homosalate, octisalate, and octinoxate. Rank orders of the in vitro and in vivo skin retention of oxybenzone and octinoxate were also comparable. Additional IVPT parameters may be optimized to enhance the discriminatory power for UV filters with low skin permeation potential (e.g., avobenzone and octocrylene).
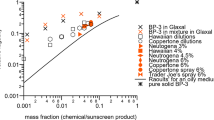
Graphical abstract





Similar content being viewed by others
References
FDA: Sunscreen Drug Products For Over-The-Counter Human Use; Final Monograph. https://www.federalregister.gov/documents/2000/06/08/00-14212/sunscreen-drug-products-for-over-the-counter-human-use-final-monograph-extension-of-effective-date (1999). Accessed 1/19/2020.
FDA: Maximal Usage Trials for Topical Active Ingredients Being Considered for Inclusion in an Over-The-Counter Monograph: Study Elements and Considerations. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM608356.pdf (2019). Accessed 01/05/2020.
Janjua NR, Kongshoj B, Andersson AM, Wulf HC. Sunscreens in human plasma and urine after repeated whole-body topical application. Journal of the European Academy of Dermatology and Venereology : JEADV. 2008;22(4):456–61. https://doi.org/10.1111/j.1468-3083.2007.02492.x.
Matta MK, Zusterzeel R, Pilli NR, Patel V, Volpe DA, Florian J, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321(21):2082–91. https://doi.org/10.1001/jama.2019.5586.
Matta MK, Florian J, Zusterzeel R, Pilli NR, Patel V, Volpe DA, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323(3):256–67. https://doi.org/10.1001/jama.2019.20747.
Calafat AM, Wong L-Y, Ye X, Reidy JA, Needham LL. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003–2004. Environ Health Perspect. 2008;116(7):893–7. https://doi.org/10.1289/ehp.11269.
Watanabe Y, Kojima H, Takeuchi S, Uramaru N, Sanoh S, Sugihara K, et al. Metabolism of UV-filter benzophenone-3 by rat and human liver microsomes and its effect on endocrine-disrupting activity. Toxicol Appl Pharmacol. 2015;282(2):119–28. https://doi.org/10.1016/j.taap.2014.12.002.
Nashev LG, Schuster D, Laggner C, Sodha S, Langer T, Wolber G, et al. The UV-filter benzophenone-1 inhibits 17beta-hydroxysteroid dehydrogenase type 3: virtual screening as a strategy to identify potential endocrine disrupting chemicals. Biochem Pharmacol. 2010;79(8):1189–99. https://doi.org/10.1016/j.bcp.2009.12.005.
Wang J, Pan L, Wu S, Lu L, Xu Y, Zhu Y, et al. Recent advances on endocrine disrupting effects of UV filters. Int J Environ Res Public Health. 2016;13(8):782. https://doi.org/10.3390/ijerph13080782.
Janjua NR, Mogensen B, Andersson AM, Petersen JH, Henriksen M, Skakkebaek NE, et al. Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. J Invest Dermatol. 2004;123(1):57–61. https://doi.org/10.1111/j.0022-202X.2004.22725.x.
Adamson AS, Shinkai K. Systemic absorption of sunscreen: balancing benefits with unknown harms. JAMA. 2020;323(3):223–4. https://doi.org/10.1001/jama.2019.20143.
Yang Y, Ako-Adounvo A-M, Wang J, Zhang J, Willett D, Yilmaz H, et al. In vitro testing of sunscreens for dermal absorption: a platform for product selection for maximal usage clinical trials. J Investig Dermatol. 2020;140:2487–95. https://doi.org/10.1016/j.jid.2020.04.009.
FDA: FDA Draft Guidance on Acyclovir Topical Cream 5%. https://www.accessdata.fda.gov/drugsatfda_docs/psg/Acyclovir_topical%20cream_RLD%2021478_RV12-16.pdf (2016). Accessed 01/10/2020.
FDA: Sunscreen Drug Products for Over-the-Counter Human Use - Proposed Rule. https://www.federalregister.gov/documents/2019/02/26/2019-03019/sunscreen-drug-products-for-over-the-counter-human-use (2019). Accessed 01/04/2020.
Kassis V, Søndergaard J. Heat-separation of normal human skin for epidermal and dermal prostaglandin analysis. Arch Dermatol Res. 1982;273(3–4):301–6. https://doi.org/10.1007/bf00409259.
Benech-Kieffer F, Wegrich P, Schaefer H. Transepidermal water loss as an integrity test for skin barrier function in vitro: assay standardization. In: Brain KR, James VJ, Walters KA, editors. Perspectives in Percutaneous Penetration. Cardiff: STS; 1997. p. 56.
Freitas JV, Praça FSG, Bentley MVLB, Gaspar LR. Trans-resveratrol and beta-carotene from sunscreens penetrate viable skin layers and reduce cutaneous penetration of UV-filters. Int J Pharm. 2015;484(1):131–7. https://doi.org/10.1016/j.ijpharm.2015.02.062.
FDA: Guidance for Industry - Bioanalytical Method Validation Guidance for Industry. https://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf (2018). Accessed 01/19/2020.
Hayton WL, Chen T. Correction of perfusate concentration for sample removal. J Pharm Sci. 1982;71(7):820–1.
Atrux-Tallau N, Pirot F, Falson F, Roberts MS, Maibach HI. Qualitative and quantitative comparison of heat separated epidermis and dermatomed skin in percutaneous absorption studies. Arch Dermatol Res. 2007;299(10):507–11. https://doi.org/10.1007/s00403-007-0789-y.
Abd E, Yousef SA, Pastore MN, Telaprolu K, Mohammed YH, Namjoshi S, et al. Skin models for the testing of transdermal drugs. Clin Pharmacol. 2016;8:163–76. https://doi.org/10.2147/CPAA.S64788.
Yuan X, Capomacchia AC. Influence of physicochemical properties on the in vitro skin permeation of the enantiomers, racemate, and eutectics of ibuprofen for enhanced transdermal drug delivery. J Pharm Sci. 2013;102(6):1957–69. https://doi.org/10.1002/jps.23548.
Acknowledgements
We are grateful to all the working group members and collaborators within the FDA who participated in the discussion of this study. Special thanks are given to Drs. Sameersingh (Sam) Raney, Priyanka Ghosh, Charles J. Ganley, Lesley-Anne Furlong, Jian Wang, Luke Oh, and Edward (Dennis) Bashaw, for their feedback on the in vitro and clinical studies. Acknowledgement extends to the late Alan S. Carlin for proofreading this manuscript.
Funding
This study was funded by the U.S. Food and Drug Administration (Maryland, USA). This project was supported in part by appointing the ORISE fellow (Dr. Ann-Marie Ako-Adounvo) to the Research Participation Program at the U.S. FDA administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. FDA.
Author information
Authors and Affiliations
Contributions
Conceptualization and design: Yang Yang, Jiang Wang, Steven A. Adah, Sergio G. Coelho.
Data curation: Yang Yang, Ann-Marie Ako-adounvo, Jiang Wang, and Muhammad Ashraf.
Investigation, methodology, and formal analysis: Yang Yang, Ann-Marie Ako-adounvo, Jiang Wang.
Writing—original draft preparation: Yang Yang, Jiang Wang.
Writing—review and editing: Yang Yang, Jiang Wang, Steven A. Adah, Sergio G. Coelho, Murali K. Matta, David Strauss, Jian Wang, Patrick J. Faustino, Muhammad Ashraf, and Thomas O’Connor.
Corresponding author
Ethics declarations
Disclaimer
This manuscript reflects the views of the authors and should not be construed to represent the views or policies of the Food and Drug Administration.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Y., Ako-Adounvo, AM., Wang, J. et al. In Vitro Testing of Sunscreens for Dermal Absorption: Method Comparison and Rank Order Correlation with In Vivo Absorption. AAPS PharmSciTech 23, 121 (2022). https://doi.org/10.1208/s12249-022-02275-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02275-z