Abstract
An accurate prediction of major adverse events after percutaneous coronary intervention (PCI) improves clinical decisions and specific interventions. To determine whether machine learning (ML) techniques predict peri-PCI adverse events [acute kidney injury (AKI), bleeding, and in-hospital mortality] with better discrimination or calibration than the National Cardiovascular Data Registry (NCDR-CathPCI) risk scores, we developed logistic regression and gradient descent boosting (XGBoost) models for each outcome using data from a prospective, all-comer, multicenter registry that enrolled consecutive coronary artery disease patients undergoing PCI in Japan between 2008 and 2020. The NCDR-CathPCI risk scores demonstrated good discrimination for each outcome (C-statistics of 0.82, 0.76, and 0.95 for AKI, bleeding, and in-hospital mortality) with considerable calibration. Compared with the NCDR-CathPCI risk scores, the XGBoost models modestly improved discrimination for AKI and bleeding (C-statistics of 0.84 in AKI, and 0.79 in bleeding) but not for in-hospital mortality (C-statistics of 0.96). The calibration plot demonstrated that the XGBoost model overestimated the risk for in-hospital mortality in low-risk patients. All of the original NCDR-CathPCI risk scores for adverse periprocedural events showed adequate discrimination and calibration within our cohort. When using the ML-based technique, however, the improvement in the overall risk prediction was minimal.
Similar content being viewed by others
Introduction
Percutaneous coronary intervention (PCI) for patients with coronary artery disease (CAD) has become widely performed1. While advances in devices and treatment strategies, residual risks of periprocedural adverse events such as acute kidney injury (AKI), bleeding, and death, remain2,3. Therefore, accurate and easy-to-use risk stratification tools for estimating the risk of these complications can provide a basis for shared decision-making and specific interventions such as bleeding avoidance strategies. For example, The United States National Cardiovascular Data Registry (NCDR) has developed risk scores (NCDR-CathPCI risk score) for periprocedural adverse events using a traditional logistic regression (LR) model with approximately 10 routinely collected preprocedural variables4,5,6, and they have been widely validated among different regions and races7.
Machine learning (ML) techniques have recently become a promising alternative approach for clinical decision support, especially in non-structured highly complex data. In fact, the number of publications focusing on ML in cardiology research has been increasing (up to 1 out of every 1,000 new publications in 2020), and the United States Food and Drug Administration has already approved a number of ML products for use in cardiology8. However, when using data from the structured electronic health record, whether the ML models improve the prediction performance of adverse periprocedural events compared to the classical LR model such as NCDR-CathPCI risk scores remains unknown. The aims of this study were (1) to evaluate the performance of the NCDR-CathPCI models in Japanese patients with CAD who underwent PCI, (2) to develop LR based and modern ML-based models using the same variables as the NCDR-CathPCI models, and (3) to compare the individual performances of the original NCDR-CathPCI, LR-based, and ML-based models.
Methods
Data source
The Japan Cardiovascular Database-Keio Interhospital Cardiovascular Studies (JCD-KiCS) is a large, ongoing, prospective multicenter (n = 15) PCI registry to collect clinical data of consecutive patients undergoing PCI in Japan that developed in collaboration with the National Cardiovascular Data Registry (NCDR) CathPCI9,10,11. In JCD-KiCS, all PCI procedures were conducted under the direction of the intervention team of each participating hospital according to standard care. Participating hospitals were instructed to register data from consecutive PCI using an electronic data-capturing software system equipped with a data query engine and validations to maintain data quality. Data entry was conducted by dedicated clinical research coordinators who trained for JCD-KiCS specifically. Data quality was ensured through the use of an automatic validation system and bimonthly standardized education and training for the clinical research coordinators. The senior study coordinator (I.U.) and extensive on-site auditing by the investigator (S.K.) ensured proper registration of each patient. The protocol of this study was under the principles of the Declaration of Helsinki and approved by the Keio University School of Medicine Ethics Committee and the committee of each participating hospital (National Hospital Organization Review Board for Clinical Trials; the Eiju General Hospital Ethics Committee; the Ethics Committee of Saiseikai Utsunomiya Hospital; the Research Ethics Committee, Tokyo Saiseikai Central Hospital; the Japanese Red Cross Ashikaga Hospital Ethics Committee; Kawasaki Municipal Hospital Institutional Review Board; Saitama City Hospital Ethical Review Board; Isehara Kyodo Hospital Institutional Review Board; Tokyo Dental College Ichikawa General Hospital Institutional Review Board; the Independent Ethics Committee of Hiratsuka City Hospital; The Saint Luke’s Health System Institutional Review Board; the Hino Municipal Hospital Institutional Review Board; and the Ethics Committee of Yokohama Municipal Citizen’s Hospital). All participants were provided verbal or written consent for the baseline data collection, and informed consent was obtained from all participants individually.
Study population
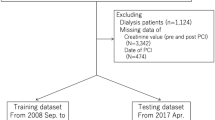
We extracted 24,848 consecutive patients who underwent PCI between July 2008 and September 2020. Because several parameters are applied as input variables for one model and the exclusion criteria of other models (e.g., hemodialysis before PCI is an input variable of the in-hospital mortality model and exclusion criteria of the AKI model), we made each outcome-specific cohort using a two-step procedure. First, we excluded patients with missing indications (n = 967), those without pre- and post-procedure hemoglobin (n = 901), and those without pre- and post-procedure serum creatinine (n = 22) (analytic cohort). Next, we applied outcome-specific exclusion criteria, followed by the imputation of missing values to make each cohort (detailed in Fig. 1). Each population was randomly split into a training set of 75% of the patients and a test set of the remaining 25% of the patients with approximately the same proportion of events.
Study flowchart. Abbreviations: CAD, coronary artery disease; PCI, percutaneous coronary intervention; JCD-KiCS, The Japan Cardiovascular Database-Keio Interhospital Cardiovascular Studies; Hb, aemoglobin; Cr, creatinine; AKI, acute kidney injury; LR logistic regression model; XGB, extreme gradient boosting model.
Definitions and outcomes
The definition of AKI, bleeding, and in-hospital mortality were consistent with original NCDR-CathPCI models4,5,6. Briefly, AKI was defined as a ≥ 0.3 mg/dl absolute or as a ≥ 1.5-fold relative increase in post-PCI creatinine or new dialysis initiation. Bleeding was defined as any of the following occurring within 72 h after PCI or before hospital discharge (whichever occurs first): site-reported arterial access site bleeding; retroperitoneal, gastrointestinal, genitourinary bleeding, intracranial hemorrhage, cardiac tamponade, or post-procedure hemoglobin decrease of 3 g/dl in patients with pre-procedure haemoglobin ≤ 16 g/dl, or post-procedure non-bypass surgery-related blood transfusion for patients with a pre-procedure haemoglobin ≥ 8 g/dl. In-hospital mortality was defined as any post-procedural death at the same hospital admission. Because JCD-KiCS was developed in collaboration with NCDR-Cath PCI, the majority of clinical variables were defined in accord with the data dictionary (version 4.1)9. For example, cardiogenic shock was defined as a sustained (> 30 min) episode of systolic blood pressure of < 90 mm Hg, and/or cardiac index of < 2.2 L/min/m2 determined to be secondary to cardiac dysfunction, and/or the requirement for intravenous inotropic or vasopressor agents or mechanical support to maintain the blood pressure and cardiac index above the specified levels within 24 h after the procedure.
Handling missing data
After enrollment of the analytic cohort, we imputed the missing value of pre-procedural hemoglobin with the value of post-procedural hemoglobin for the developed AKI and in-hospital mortality model, and imputed missing values of pre-procedural creatinine with those of post-procedural creatinine for the developed bleeding and in-hospital mortality models. Given that the absence rate was < 5% for any other variables, we handled the missing values to use a median imputation for the continuous variables and mode imputation for the categorical variables.
Model development
We developed two models: LR models and extreme gradient descent boosting (XGB) models. XGB is an ML algorithm that creates a series of relatively simple decision trees combined with boosting methods to develop more robust final predictions. In the LR model, we used the same categorized variables of the original NCDR-CathPCI risk scores (original model), and in the XGB model, we used the same variables but treated raw continuous variables that were categorized in the original models. The full list of variables was as follows:
-
1.
AKI model: age (categorized as < 50, 50–59, 60–69, 70–79, 80–89, and ≥ 90 years), heart failure within 2 weeks, estimated glomerular filtration rate (eGFR) (categorized as < 30, 30–44, 45–59, and ≥ 60 ml/min/1.73 m2), diabetes mellitus, prior heart failure, prior cerebrovascular disease, non ST-elevation acute coronary syndrome (NSTEACS), ST-elevation myocardial infarction (STEMI), cardiogenic shock at presentation, cardiopulmonary arrest at presentation, anemia defined as hemoglobin at admission of less than 10 g/dL, and use of IABP.
-
2.
Bleeding model: STEMI, age (categorized as < 60, 60–70, 71–79, and ≥ 80 years), BMI (categorized as < 20, 20–30, 30–39, and ≥ 40 kg/m2), prior PCI, eGFR (categorized as < 30, 30–44, 45–59, and ≥ 60 ml/min/1.73 m2), cardiogenic shock at presentation, female sex, hemoglobin at presentation (categorized as < 13, 13–15, ≥ 15 g/dL), and PCI status (Emergency, Salvage, Urgency, and Elective).
-
3.
In-hospital mortality model: age (categorized as < 60, 60–69, 70–79, and ≥ 80 years), cardiogenic shock at presentation, prior heart failure, peripheral artery disease, chronic obstructive pulmonary disease, estimated GFR (categorized as < 30, 30–44, 45–59, 60–89, and ≥ 90 ml/min/1.73 m2), NYHA classification IV at presentation, STEMI, and PCI status (emergency, salvage, urgency, and elective).
To optimize the hyperparameters of the XGB model, we used a stratified threefold cross-validation with a random search. After determining the best hyperparameters, XGB models were developed using the entire training set (hold-out methods, Supplementary Material for a more detailed explanation). In addition, we constructed the expanded LR and XGB models using additional variables selected by clinical significance. The additional variables were as follows:
-
Expanded AKI model: contrast volume and timing of PCI (i.e., during working or holiday times).
-
Expanded bleeding model: number of antiplatelet agents, use of anticoagulants at PCI, and timing of PCI.
-
Expanded in-hospital mortality model: technical failure of PCI, defined as failure to cross the guidewires or when the TIMI grade after PCI was 1 or 0 (slow flow or no flow), and the timing of PCI.
Statistics and key metrics
Continuous variables were summarized as medians with interquartile ranges and compared using Mann–Whitney U tests, and categorical variables were summarized as frequencies and compared using chi-square tests or Fisher’s exact tests, as appropriate.
The C-statistics with 95% confidence intervals (95%CIs) based on the Delong method and the area under the precision-recall area under curve (PRAUC) were used to estimate the model discrimination. Model calibration was assessed using the Brier score and calibration plot. The Brier score is defined as the mean squared difference between the observed and predicted outcomes and ranges from 0 to 1.00, with 0 representing the best possible calibration. The two primary components decomposed from the Brier score, i.e., reliability and resolution, were also evaluated. Calibration plots were used to plot the mean risk score relative to the observed outcome rate for a given quintile of the predicted risk. Furthermore, we used the net reclassification index (NRI) to evaluate the clinical utility of the LR and XGB models with cut-off values of 10%, 4%, and 2.5% for AKI, bleeding, and in-hospital mortality, respectively. A P value of < 0.05 was considered statistically significant. This study is based on the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) guidelines.
Sensitivity analysis
We used a multiple imputation method to handle missing values instead of a median imputation method. The multiple imputation model included all prespecified predictors and outcomes as recommended12. Ten imputed datasets were generated, and the C-statistics were combined using Rubin’s rules.
Software Implementation
All analyses were conducted in R (version 4.0.4; R Project for Statistical Computing, Vienna, Austria) with tidymodels (version 0.1.2) bundle of packages for data pre-processing, hyperparameter tuning, learning, and performance metrics13,14,15. We used xgboost (version 1.3.2.1) for extreme gradient descent boosting16, pROC (version 1.17.0.1) to calculating C-statistics17, verification (version 1.42) to calculate Brier scores18, predictABEL (version 1.2.4) to calculate the NRI19 mice (version 3.14.0) to perform multiple imputation20.
Results
Patient characteristics
Between July 2008 and September 2020, a total of 22,958 consecutive patients with CAD who underwent PCI were analyzed. The patients were predominantly men with a median age of 70 (interquartile range [IQR] 62, 77) years, and a body mass index of 24.0 (21.9, 26.3). Overall, 55.4% of the patients had stable ischemic heart disease, and 58.6% underwent elective PCI. The prevalence of AKI, bleeding, and in-hospital mortality were 9.6%, 7.8%, and 2.3%, respectively (Table 1). The baseline characteristics of patients in training and test set of each outcome were in Table S1 from Supplementary Material.
Model discrimination
The original models for each outcome showed good discrimination (C-statistics of 0.82, 95% CI [0.80–0.84] for AKI; C-statistics of 0.76, 95% CI [0.73–0.78] for bleeding; C-statistics of 0.95, 95% CI [0.94–0.97] for in-hospital mortality). The LR model modestly improved the discrimination in AKI (C-statistics of 0.83, 95% CI [0.81–0.85], P = 0.04). The XGB models also modestly improve the discrimination in AKI and bleeding (C-statistics of 0.84, 95% CI [0.82–0.86], P < 0.001 for AKI; C-statistics of 0.79, 95% CI [0.76–0.81], P < 0.001 for bleeding) but not in-hospital mortality (Fig. 2). The performance of each model, including PRAUC, was presented in Table 2. Further, the expanded models did not improve discrimination over the original models (Table S3). Using a multiple imputation dataset, the main results were consistent with the main findings (Table S4).
Model calibration
In the original models, the calibration was adequate for each outcome (Brier score of 0.064 for AKI, 0.087 for bleeding, and 0.021 for in-hospital mortality). Whereas XGB models and LR models showed equivalent to the original models for each outcome in the Brier score and its components, the calibration plot showed an overestimated in-hospital mortality in low-risk patients (Fig. 3). The patients in the first and second quintile of the XGB model were likely to be elective cases with SIHD for PCI indication, and no patient presented with cardiogenic shock. Notably, there were no in-hospital deaths among these low-risk patients. The discrimination and calibration of the original models for the total cohort are shown in Table S2.
Model reclassification
Compared with the original models, the LR models improved the reclassification for AKI, whereas no difference was observed in the bleeding, and a decline in the net reclassification index was shown in the in-hospital mortality. The XGB models improved the reclassification of AKI and bleeding but declined the reclassification for in-hospital death (Table 3).
Discussion
Using a Japanese multicenter PCI registry that was constructed in-sync with NCDR, we demonstrated: (1) The original NCDR CathPCI risk scores for predicting the incidence of each outcome showed a considerable performance in terms of the discrimination and calibration in Japan, and (2) compared with the original NCDR-CathPCI risk scores, ML models showed no or modest improvement in the discrimination and decreased calibration, particularly in-hospital mortality.
In our analysis, the C-statistics of all NCDR-CathPCI risk scores were more than 0.75, which was considered clearly useful discrimination21. While the discrimination of the ML models being better than that of the original models with a statistical significance, the absolute difference in C-statistics was minimal (0.02 in AKI and bleeding). In addition, while a sufficient calibration performance is necessary to apply in clinical practice21, the XGB model of in-hospital mortality was overestimated in patients in the low-risk category. This falsely high mortality risk may lead a patient to choose not to undergo a procedure inappropriately. Such poor calibration in ML models related to LR models is consistent with a previous study22. The plausible mechanism of overestimation in the low-risk category in in-hospital mortality might be largely owed to the low event rates observed in this group; there were no in-hospital deaths among the low-category patients. Imbalanced data pose a challenge in the machine learning field. A previous study showed that calibration performance in imbalanced data is biased because ML-based models considered the majority class to be more important than the minority class23. Furthermore, we constructed machine learning models based on the best AUROC values. This metric was known to be less sensitive to imbalanced data, and PRUAC was the preferred metric when data was imbalanced24. While AUROC has potential limitations, it was the most common metric for evaluating the prediction models and the most intuitive, whereas PRAUC did not have such a “rule of thumb21.” Considering the above, caution is required when constructing ML-based models using imbalanced data. Further research is needed to construct ML-based models for the imbalanced data.
ML techniques are data-driven and do not require several assumptions, whereas LR models are theory-driven and require several assumptions such as data distribution, variance equality, and linearity. Owing to freedom from these assumptions, ML models can handle non-linearity associations and interactions naturally25. Therefore, ML models are useful when the outcome and input variables have a complex relationship. A previous study showed that a gradient boosting model with age, sex, and paired high-sensitivity cardiac troponin-I (hs-TnI) showed better performance in predicting myocardial infarction (AUROC of 0.963 [0.956–0.971] in early and late presentation) than the ESC 0/3 h pathway26. ML techniques, such as deep neural networking algorithms, have shown excellent performance when dealing with high-dimensional, highly self-correlated data such as medical imaging that could not be dealt with classic statistical models27. Furthermore, the ML technique can recognize negligible change that humans cannot in time-dependent continuous variables, such as in electrocardiograms. Indeed, the ML technique can identify the reduced ejection fraction or hypertrophic cardiomyopathy27,28.
Otherwise, when dealing with fewer weakly correlated clinical variables such as structured electronic health records, LR models are likely to perform as well as ML models29. A systematic review showed no difference in discrimination between ML-based and LR-based models when using research with a low risk of bias30.
Beyond the simple measurement of performance, it is important to account for the deployment and maintenance of risk models. Both of them are difficult in ML-based models due to their insufficiency of explainability and risk for overfitting31, whereas LR models such as the NCDR-risk scores could easily implement and update. For example, pre/post-implementation studies have shown that integrating a stratification by the NCDR-CathPCI bleeding model and using a bleeding avoidance strategy can reduce periprocedural bleeding32. Further, NCDR-risk scores have been updated when concerns are raised5,33,34. Considering the above, it would be difficult to justify using ML-based models instead of NCDR-CathPCI risk scores within our cohort. Future analyses are needed to determine whether LR or ML-based models are better for specific data structures and outcomes.
There are several limitations to this study. First, we did not conduct an external validation for the LR and XGB models. However, we first separated the test sets to avoid leakage as recommended35, and were no registries that collaborated with NCDR-CathPCI in Japan except for JCD-KiCS. Second, we did not modify input variables. The input variables in original risk scores were selected based on the correlation and backward elimination methods using a logistic regression model. Otherwise, XGB models can use an embedded feature selection using variable importance36. XGB models with the other variables may improve the performance. However, the variables we used were clinically acceptable and intuitive. Finally, we did not develop other ML models, such as support vector machines and neural networks. However, previous studies have shown that the XGB algorithm performs better than those algorithms in cardiology research37.
Conclusion
All of the original NCDR-CathPCI risk scores for adverse periprocedural events showed adequate discrimination and calibration within our cohort. The discrimination of bleeding and AKI risk improved modestly when ML-based models were incorporated; however, the improvement in the overall risk prediction was minimal.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Virani, S. S. et al. Heart disease and stroke statistics—2021 update. Circulation 143, e254–e743 (2021).
Amin, A. P. et al. Trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction. Arch. Intern. Med. 172, 246–253 (2012).
Subherwal, S. et al. Temporal trends in and factors associated with bleeding complications among patients undergoing percutaneous coronary intervention: a report from the National Cardiovascular Data CathPCI Registry. J. Am. Coll. Cardiol. 59, 1861–1869 (2012).
Tsai, T. T. et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath-PCI registry. J. Am. Heart Assoc. 3, 1–13 (2014).
Rao, S. V. et al. An updated bleeding model to predict the risk of post-procedure bleeding among patients undergoing percutaneous coronary intervention: a report using an expanded bleeding definition from the national cardiovascular data registry CathPCI registry. JACC Cardiovasc. Interv. 6, 897–904 (2013).
Petersen, E. D., Dai, D. & Delong, E. R. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588, 398 procedures in the national cardiovascular data registry. J Am Coll Cardiol. 29, 1767–1770 (2010).
Wolff, G. et al. Validation of National Cardiovascular Data Registry risk models for mortality, bleeding and acute kidney injury in interventional cardiology at a German Heart Center. Clin. Res. Cardiol. 109, 235–245 (2020).
Quer, G., Arnaout, R., Henne, M. & Arnaout, R. Machine learning and the future of cardiovascular care: JACC state-of-the-art review. J. Am. Coll. Cardiol. 77, 300–313 (2021).
Kohsaka, S. et al. An international comparison of patients undergoing percutaneous coronary intervention: a collaborative study of the National Cardiovascular Data Registry (NCDR) and Japan Cardiovascular Database-Keio interhospital Cardiovascular Studies (JCD-KiCS). Am. Heart J. 170, 1077–1085 (2015).
Inohara, T. et al. Performance and Validation of the U.S. NCDR Acute Kidney Injury Prediction Model in Japan. J. Am. Coll. Cardiol. 67, 1715–1722 (2016).
Inohara, T. et al. Use of intra-aortic balloon pump in a Japanese multicenter percutaneous coronary intervention registry. JAMA Intern. Med. 175, 1980–1982 (2015).
Sterne, J. A. C. et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 338, b2393 (2009).
R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (2021).
Wickham, H. et al. Welcome to the tidyverse. J. Open Source Softw. 4, 1686 (2019).
Kuhn, M. & Wickham, H. Tidymodels: a collection of packages for modeling and machine learning using tidyverse principles. https://www.tidymodels.org (2020).
Chen, T. et al. xgboost: Extreme Gradient Boosting. https://github.com/dmlc/xgboost (2021).
Robin, X. et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 12, 77 (2011).
- Research Applications Laboratory, N. verification: Weather Forecast Verification Utilities. https://CRAN.R-project.org/package=verification (2015).
Kundu, S., Aulchenko, Y. S. & Janssens, A. C. J. W. PredictABEL: Assessment of Risk Prediction Models. https://CRAN.R-project.org/package=PredictABEL (2020).
van Buuren, S. & Groothuis-Oudshoorn, K. mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011).
Alba, A. C. et al. Discrimination and calibration of clinical prediction models: users’ guides to the medical literature. JAMA 318, 1377–1384 (2017).
Niculescu-Mizil, A. & Caruana, R. Predicting good probabilities with supervised learning. in Proceedings of the 22nd international conference on Machine learning - ICML ’05 (ACM Press, 2005). doi:https://doi.org/10.1145/1102351.1102430.
Wallace, B. C. & Dahabreh, I. J. Improving class probability estimates for imbalanced data. Knowl. Inf. Syst. 41, 33–52 (2014).
Saito, T. & Rehmsmeier, M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS One 10, e0118432 (2015).
Goldstein, B. A., Navar, A. M. & Carter, R. E. Moving beyond regression techniques in cardiovascular risk prediction: applying machine learning to address analytic challenges. Eur. Heart J. 38, 1805–1814 (2017).
Than, M. P. et al. Machine learning to predict the likelihood of acute myocardial infarction. Circulation 899–909 (2019).
Attia, Z. I. et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 394, 861–867 (2019).
Ko, W.-Y. et al. Detection of hypertrophic cardiomyopathy using a convolutional neural network-enabled electrocardiogram. J. Am. Coll. Cardiol. 75, 722–733 (2020).
Engelhard, M. M., Navar, A. M. & Pencina, M. J. Incremental benefits of machine learning—when do we need a better mousetrap?. JAMA Cardiol https://doi.org/10.1001/jamacardio.2021.0139 (2021).
Christodoulou, E. et al. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J. Clin. Epidemiol. 110, 12–22 (2019).
Pencina, M. J., Goldstein, B. A. & D’Agostino, R. B. Prediction models—development, evaluation, and clinical application. N. Engl. J. Med. 382, 1583–1586 (2020).
Spertus, J. A. et al. Precision medicine to improve use of bleeding avoidance strategies and reduce bleeding in patients undergoing percutaneous coronary intervention: prospective cohort study before and after implementation of personalized bleeding risks. BMJ 350, h1302–h1302 (2015).
Brennan, J. M. et al. Enhanced mortality risk prediction with a focus on high-risk percutaneous coronary intervention: results from 1,208,137 procedures in the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc. Interv. 6, 790–799 (2013).
Castro-Dominguez, Y. S. et al. Predicting In-Hospital Mortality in Patients Undergoing Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. https://doi.org/10.1016/j.jacc.2021.04.067 (2021).
Stevens, L. M., Mortazavi, B. J., Deo, R. C., Curtis, L. & Kao, D. P. Recommendations for reporting machine learning analyses in clinical research. Circ. Cardiovasc. Qual. Outcomes 782–793 (2020).
Al’Aref, S. J. et al. Determinants of in-hospital mortality after percutaneous coronary intervention: a machine learning approach. J. Am. Heart Assoc. 8, (2019).
Khera, R. et al. Use of machine learning models to predict death after acute myocardial infarction. JAMA Cardiol https://doi.org/10.1001/jamacardio.2021.0122 (2021).
Acknowledgements
We would like to thank all study coordinators, investigators, and the patients who participated in the JCD–KiCS registry. The present study was funded by the Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI; Nos. 16KK0186, 16H05215, 18K17332, and 20H03915 https://kaken.nii.ac.jp/ja/index/). We also would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
N.N., Y.S. and S.K. designed the experiment. N.N. performed the ML and statistical analysis. N.N., Y.S., N.I., T.I. and S.K. interpreted the results. N.N., M.S., T.I., I.U., and S.K. contributed with data and respective curation. I.U., K.F., and S.K. administered and technical support. K.F. and S.K. obtained funding. All authors reviewed and critical revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Shiraishi is affiliated with an endowed department by Nippon Shinyaku Co., Ltd., Medtronic Japan Co., Ltd., and BIOTRONIK JAPAN Inc., and received research grants from the SECOM Science and Technology Foundation and the Uehara Memorial Foundation and honoraria from Otsuka Pharmaceuticals Co., Ltd. and Ono Pharmaceuticals Co., Ltd.; Dr. Kohsaka received an unrestricted research grant from the Department of Cardiology, Keio University School of Medicine, Bayer Pharmaceutical and Pfizer Japan. The other authors declare no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Niimi, N., Shiraishi, Y., Sawano, M. et al. Machine learning models for prediction of adverse events after percutaneous coronary intervention. Sci Rep 12, 6262 (2022). https://doi.org/10.1038/s41598-022-10346-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-10346-1
This article is cited by
-
Using the Super Learner algorithm to predict risk of major adverse cardiovascular events after percutaneous coronary intervention in patients with myocardial infarction
BMC Medical Research Methodology (2024)
-
Predictive modeling for acute kidney injury after percutaneous coronary intervention in patients with acute coronary syndrome: a machine learning approach
European Journal of Medical Research (2024)
-
Contemporary Risk Models for In-Hospital and 30-Day Mortality After Percutaneous Coronary Intervention
Current Cardiology Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.