Abstract
Background
Crizotinib was the first oral targeted therapy approved by the US Food and Drug Administration (FDA), on 11 March 2016, for c-ros oncogene 1 (ROS1)-positive advanced non-small-cell lung cancer (NSCLC). Data to support long-term clinical benefit in a real-world setting are limited.
Objective
This study aimed to assess real-world clinical outcomes among patients with ROS1-positive advanced NSCLC treated with crizotinib in the US community oncology setting.
Patients and Methods
We conducted a retrospective cohort study using iKnowMed electronic health record data to identify adult patients with ROS1-positive advanced NSCLC who initiated crizotinib between 17 January 2013 (time of the addition of crizotinib for ROS1-positive NSCLC to National Comprehensive Cancer Network (NCCN) treatment guidelines) and 1 June 2019 with a potential follow-up period through 1 December 2019. Patient characteristics were assessed descriptively. Kaplan–Meier analyses were used to evaluate time to treatment discontinuation (TTD), time to next treatment (TTNT), and overall survival (OS). A Cox proportional hazards model was conducted to determine factors associated with OS.
Results
The study cohort included 38 ROS1-positive patients treated with crizotinib. The median age was 68 years (interquartile range 60.0–73.0) and 65.8% were female. Over 50% were current/former smokers, and 18.4% had an Eastern Cooperative Oncology Group (ECOG) performance status of 2. Overall, 21 (55.3%) patients remained on crizotinib, 10 (26.3%) had evidence of subsequent treatment, and 16 (42.1%) died. The median TTD, TTNT, and OS were 25.2 months [95% confidence interval (CI): 5.2–not reached (NR)], 25.0 months (95% CI 5.2–61.0), and 36.2 months (95% CI 15.9–NR), respectively. In a multivariate Cox regression model, ECOG performance status of 2 was associated with a 4.9-fold higher risk of death (hazard ratio = 4.9; 95% CI 1.1–21.4) compared to ECOG performance status of 0 or 1.
Conclusions
This ROS1-positive NSCLC real-world population was older and had a higher proportion of smokers and of patients with poorer ECOG performance status than those investigated in clinical trials. Nevertheless, our findings support the clinical benefit of crizotinib in this patient population with ROS1-positive advanced NSCLC.
Similar content being viewed by others
This study characterizes the real-world outcomes for patients with ROS1-positive advanced NSCLC treated with crizotinib. |
Patients with ROS1-positive advanced NSCLC who received crizotinib had poor prognostic patient characteristics compared to clinical trials. |
Our study found median overall survival of 36.2 months, which was shorter than PROFILE 1001 but similar to East Asian (OO12-1) clinical trials. |
1 Introduction
Lung cancer is the second most common cancer and the leading cause of cancer-related mortality in the USA. In 2021, an estimated 235,760 new cases of lung cancer will be diagnosed with about 131,880 deaths from the disease [1, 2]. Lung cancer is more common in smokers, women, and people aged 65 years and older. Non-small-cell lung cancer (NSCLC) accounts for 80–85% of all lung cancers [1, 2] and approximately half of those patients present with Stage IV disease [1, 2]. The overall 5-year survival rate is 24.6% for all patients with NSCLC and 6.1% for those diagnosed with stage IV (distant) NSCLC [1]. In the USA, population-level mortality from NSCLC fell sharply from 2013 to 2016, partially driven by improvements in the treatments for oncogenic driver mutations [3].
Several genomic alterations have been identified as biomarkers for cancer detection, diagnosis, and prognosis in NSCLC, such as anaplastic lymphoma kinase (ALK) gene rearrangements, c-ros oncogene 1 (ROS1) gene rearrangements, sensitizing epidermal growth factor receptor (EGFR) mutations, v-raf murine sarcoma viral oncogene homolog B1 (BRAF) point mutations, gene Kirsten rat sarcoma viral oncogene homolog (KRAS), and programmed death-ligand 1 (PD-L1) expression [4]. ROS1 rearrangements occur in 1–2% of patients with NSCLC [5, 6].
Crizotinib is an oral multitargeted tyrosine kinase inhibitor (TKI) that targets the ALK, ROS1, mesenchymal epithelial transition factor receptor (MET), and Recepteur d’Origine Nantais (RON) biomarkers [7,8,9]. The National Comprehensive Cancer Network (NCCN) guideline has recommended crizotinib for ROS1-positive NSCLC since January 2013 [10] and the US Food and Drug Administration (FDA) approved crizotinib for ROS1-positive NSCLC on 11 March 2016 [11]. The ROS1 approval was based on a Phase I single-arm study (PROFILE 1001) that showed an investigator-assessed objective response rate (ORR) of 72% (95% confidence interval (CI) 58–84), independent radiology review (IRR) ORR of 66% (95% CI 51–79), and IRR median duration of response (DoR) of 17.6 months [11]. Clinical trials from East Asia (OO1201) [12, 13], Europe (EUCROSS) [14], and Italy (METROS) [15] also demonstrated efficacy of crizotinib for patients with ROS1-positive NSCLC.
There is a paucity of research, particularly in the US population, that describes the clinical profile of patients with ROS1-positive advanced NSCLC receiving crizotinib and associated clinical outcomes in a real-world setting [16,17,18]. Understanding the effectiveness of crizotinib in the US population will help clinicians and patients make informed treatment decisions. In this study, using electronic health record (EHR) data from US community oncology practices, we aimed to describe patient characteristics and clinical outcomes among patients with ROS1-positive advanced NSCLC treated with crizotinib.
2 Methods
2.1 Data Source
We utilized iKnowMed™ (iKM) EHR data maintained by McKesson Specialty Health (MSH) from 17 January 2013 to 1 December 2019. iKM is an oncology-specific EHR system that is implemented across the US Oncology Network and other non-Network community oncology practices. It captures outpatient practice encounter histories for patients under community-based care, including (but not limited to) patient demographics such as age and sex; clinical information such as disease diagnosis, diagnosis stage, and laboratory testing results; treatment information such as lines of therapy and treatment administration; comorbidities; and performance status. The US Oncology Network includes approximately 1400 affiliated physicians operating in more than 480 sites of care across 25 states and treats approximately 1 million patients with cancer in the USA annually. There are approximately 80 non-Network practices that utilize the iKM EHR. Overall, the iKM EHR system captures data on approximately 10% of patients with newly diagnosed cancer in the USA. Structured data from the iKM database were used to address the research questions in this study. Data were obtained via programmatic database abstraction with supplemental vital status provided by the Social Security Administration’s Limited Death Access Master File (LADMF).
LADMF includes records of deaths reported by family members, funeral homes, hospitals, financial institutions, postal authorities, and federal agencies for persons issued a Social Security card. A study comparing the accuracy of death data between iKM EHR and LADMF reported a concordance of 88.0%, with 93.3% of the death data being captured from the structured data of the iKM EHR while LADMF supplied an additional 6.7% [19].
The study protocol received an exception and waiver of informed consent from the US Oncology Institutional Review Board. The security of the data meets the requirements of the Health Insurance Portability and Accountability Act of 1996, and the study adheres to the principles outlined in the Declaration of Helsinki.
2.2 Study Design and Patient Population
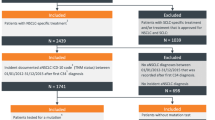
This retrospective cohort study included patients with ROS1-positive advanced NSCLC who initiated crizotinib between 17 January 2013 and 1 June 2019 (i.e., study identification period) within the eligible practices utilizing the iKM EHR. The date 17 January 2013 was selected as the start date based on the date when NCCN guidelines first recommended crizotinib for patients with ROS1-positive NSCLC. Diagnosis of NSCLC was determined through a review of iKM’s discrete diagnosis and histology fields, which were populated during the routine course of care.
Patients were included in the study cohort if they met the following criteria: (i) age ≥ 18 years at initial diagnosis of NSCLC, (ii) received crizotinib during the study identification period, (iii) had ROS1 testing results documented as positive in the structured field of EHR (quantitative measure of biomarker positivity were not available in the structured EHR data), and (iv) had two or more clinic visits within the eligible practices following diagnosis during the study observation period. The first prescription of crizotinib was considered as the index date. Patients were followed longitudinally until 1 December 2019 (i.e., end of study observation period), last patient record, or date of death, whichever occurred first. Figure 1 in the Electronic Supplementary Material (ESM) describes the study design.
2.3 Clinical Outcomes
Time to treatment discontinuation (TTD) was defined as the interval between index treatment initiation and permanent treatment discontinuation within the study observation period for any cause. TTD included an assumption of 30 days’ supply from the last observed dose in the EHR. Patients who did not discontinue treatment during the study observation period were censored on the study end date or the last visit date available in the dataset, whichever occurred first. Time to next treatment (TTNT) was measured from index treatment initiation to the start date of the next treatment or date of death due to any cause; patients were censored if they did not receive a subsequent treatment by the end of the study observation period. Overall survival (OS) was defined as the interval between the index treatment and the date of death due to any cause as documented in the LADMF and the iKM EHR database. Patients who did not die within the study observation period were censored on the study end date or the last visit date available in the dataset, whichever occurred first. All time-to-event outcomes were provided in months.
2.4 Patient Characteristics
Patient characteristics were collected on the index date or at the closest date prior to the index date within 30 days. Demographic characteristics included age, sex, race, body mass index (BMI), geographic location, smoking history, and payer type. Clinical characteristics included advanced NSCLC diagnosis year, stage at diagnosis, Eastern Cooperative Oncology Group (ECOG) performance status, tumor histology, distant metastatic site(s), count of metastatic site(s), ROS1 rearrangements, ALK rearrangements, EGFR mutations, PD-L1 expression, BRAF mutations, and Charlson comorbidities. Any systemic anticancer therapy received before and after the initiation of crizotinib was also captured.
2.5 Statistical Analysis
Descriptive statistics were conducted to describe the demographic profiles, clinical characteristics, overall follow-up period, and treatment patterns for the study cohort. TTD, TTNT, and OS were assessed using the Kaplan–Meier method and the restricted mean survival time (RMST). TTNT was also assessed using Fine and Gray’s competing risk model to account for competing risk of death [20]. A Cox proportional hazard regression model was constructed for OS and included age, smoking status, BMI, ECOG performance status, and duration of time from initial NSCLC diagnosis to the index treatment as independent variables. All analyses were conducted using SAS 9.4 (SAS Inc, Cary, NC, USA).
3 Results
Across all participating practices utilizing the full EHR capacities, more than 154,000 patients with a diagnosis of NSCLC were identified. In total, 154,511 patients were excluded because they were aged < 18 years (n = 168), did not receive crizotinib during the study identification period (n = 153,549), or had ROS1 status of negative or not documented (n = 794) (Fig. 1). In the final cohort, 38 patients met eligibility criteria and were included in the analysis (Fig. 1). The median follow-up time from crizotinib initiation to the end of the study observation period or death was 15.3 (interquartile range (IQR) 4.5–29.4) months. Among all 38 patients, 76.3% received crizotinib therapy as first-line (1L) and 23.7% received it as second-line (2L) or greater.
The median age of the cohort was 68.0 years (IQR 60.0–73.0), with 65.8% female and 71.1% White (Table 1). The largest proportions of patients were treated in the Midwest/Northeast (42.1%) and West (31.6%) regions of the USA. More than half of the patients were current/former smokers (55.9%) and had a median BMI of 25.2 kg/m2 (IQR 21.2–30.7) with the majority being overweight or obese (57.2%) (Table 1). The largest proportion of patients were diagnosed with stage IV disease at diagnosis (67.6%) and had non-squamous histology (76.3%). Approximately one in five (18.4%) patients had an ECOG performance status of 2 and three-fifths (63.1%) of patients had at least one comorbidity. Most patients initiated crizotinib in 2016 or later (86.8%). Approximately one-third (34.2%) of patients received systemic anticancer treatment prior to crizotinib and 26.4% received systemic anticancer treatment after crizotinib (Table 2).
3.1 Time to Treatment Discontinuation (TTD)
Overall, 21 (55.3%) patients remained on crizotinib through the end of the study observation period. Kaplan–Meier analysis revealed that the median TTD was 25.2 months (95% CI 5.2–not reached (NR); Fig. 2A), and crizotinib discontinuation probabilities at 6, 12, and 24 months were 32.6%, 43.5%, and 47.8%, respectively. The estimated RMST for TTD at 42 months of follow-up for crizotinib users was 21.9 months (95% CI 15.7–28.0). This indicates that patients receiving crizotinib followed for 42 months would discontinue crizotinib at an average of 21.9 months.
Kaplan–Meier curves for clinical outcomes among patients with ROS1-positive advanced NSCLC receiving crizotinib. a Time to treatment discontinuation (TTD)—KM curves with 95% confidence interval (CI). b Cumulative incidences for time to next treatment (TTNT)—Competing Risk Model with 95% CI. c Overall survival (OS)—KM curves with 95% CI
3.2 Time to Next Treatment (TTNT)
Ten (26.3%) patients received subsequent treatment. After accounting for competing risk of death, probabilities of remaining on crizotinib at 6, 12, and 24 months were 86.1%, 80.3%, and 77.1%, respectively (Fig. 2B). Kaplan–Meier analysis results are reported in the ESM (ESM Figure 2).
3.3 Overall Survival (OS)
Among the overall study population, 16 (42.1%) patients died during the study observation period. Median OS from crizotinib initiation was 36.2 months (95% CI 15.9–NR), and survival rates at 6, 12, and 24 months were 77.8%, 71.9%, and 64.9%, respectively (Fig. 2C). The 42-month RMST showed that patients would survive, on average, 27.3 months (95% CI 21.7–32.8) of the 42 months of follow-up.
In the Cox regression analysis, after controlling for age, smoking status, BMI, and duration between NSCLC diagnosis and crizotinib initiation, having an ECOG performance status of 2 was associated with 4.9-fold higher risk of death (HR = 4.9; 95% CI 1.1–21.4; P = 0.0337) compared to ECOG performance status of 0 or 1. Similarly, current/former smoker status was associated with 6.0-fold higher risk of death (HR = 6.0; 95% CI 1.2–30.8; P = 0.0326) compared to never smokers, after controlling for age, ECOG performance status, BMI, and duration between diagnosis and crizotinib initiation (Table 3).
4 Discussion
This real-world study provides insights into the patient characteristics and clinical outcomes among 38 patients diagnosed with ROS1-positive advanced NSCLC who received crizotinib in a US community-practice setting. Our study showed clinical effectiveness of crizotinib with a median TTD of 25.0 months, TTNT of 25.2 months, and OS of 36.2 months.
In comparison to three of the four clinical trials (PROFILE 1001 [21], East Asia (OO12-01) [12], and EUCROSS [14]) that evaluated crizotinib use in ROS1-positive advanced NSCLC, our study population was older (68 vs. range 51–56 years in clinical trials) with a predominance of smokers (56% vs. 25–32%) and ECOG performance status of 2 (18% vs. 0–6%). At the same time, the median age of our study population was comparable to patients enrolled in the METROS clinical trial (68 years) [15]. In comparison to real-world studies from Europe [16] and China [17], our population was also older (68 vs. 51–52 years) with a higher proportion of patients being smokers (56% vs. 17–32%). Although patients who are smokers tend to be under-tested despite guidelines stating that tobacco use should not be used as a predictive factor in deciding whom to test, more than half of patients were smokers in this study [22, 23]. Demographic and clinical profiles of patients in our study differed considerably from clinical trials and real-world studies from other countries highlighting variation in crizotinib patient populations across studies. Even though our study included a population with a poorer prognosis, we observed clinical benefits of crizotinib use. Previous clinical trials and real-world studies from other countries did not report TTNT but reported PFS. TTNT and to some extent TTD could be considered surrogates for PFS [24], and therefore we compared our findings with PFS from those studies. We observed higher median TTD (25.0 months) and TTNT (25.2 months) compared to median PFS from East Asia (15.9), PROFILE 1001 (19.2 months), EUCROSS (20.0 months), and METROS (22.8 months) clinical trials [12, 14, 15, 21]. Similar findings were observed in the real-world studies (median PFS 9.1–18.4 months) [16, 17]. A retrospective study from Europe (EUROS1) included 32 patients and found that patients with ROS1-positive lung cancer who received crizotinib had a median PFS of 9.1 months [16]. A study from China including 30 patients with ROS1-positive NSCLC found that patients who received first-line crizotinib had a higher median PFS than those who received platinum‐pemetrexed chemotherapy (18.4 vs. 8.6 months) [17]. The median OS in our study was 36.2 months, which was similar to the results of the East Asian (Study OO12-01) clinical trial (32.5 months) [12] and was shorter than the OS reported in PROFILE 1001 (51.4 months) [21]. Clinical outcomes could be affected by patient characteristics and therapies that were taken before or after crizotinib, but the variation in clinical outcomes due to different treatment sequences was not evaluated in this study. The follow-up time in our study is 15.3 months. The follow-up time observed in the East Asian clinical trial was 21.4 months [12], whereas it was 62.6 months in PROFILE 1001 [21]. It is possible that the OS in our study will be closer to PROFILE 1001 if our study patients had a longer follow-up period. Our study also showed that ECOG performance status of 2 and smoking status were the strongest predictors for survival, with a higher proportion of patients having these characteristics. This could explain the shorter OS observed.
The results of this study should be considered in the context of the strengths and limitations of the data source and study design. The iKM database is not collected for research purposes but for clinical practice reasons. This may have impeded the standardization of the data collection methods, instruments, and reporting practices of the physician. The iKM EHR contains information on patients only when they are seen by their community oncology physicians. Services and procedures provided outside of their clinic (e.g., hospitalizations or radiation therapies) are not captured by the database. Not all community oncology practices utilize the iKM EHR and decision-support technology. Therefore, the results of this study will be most generalizable to other community oncology practices that also adhere to evidence-based treatment guidelines. As the study only used structured data fields from the electronic health record, we were unable to assess the presence of brain metastases after crizotinib initiation or reasons for permanent treatment discontinuation. Also, it is likely that we underestimated the number of patients testing positive for the ROS1 biomarker [25]. We did not evaluate if ROS1 testing was performed for all NSCLC patients and therefore we do not know the true rate of genotyping performed among all NSCLC patients. The prevalence of ROS1 in the general NSCLC population suggests that in our population not all advanced NSCLC patients were routinely tested for the ROS1 biomarker. This implies that there is a lack of biomarker testing, potentially leading to an underrepresentation of ROS1 patients [26, 27]. Also, testing of ROS1 has evolved over the last decade from predominantly fluorescence in situ hybridization (FISH)-based assays to next-generation sequencing (NGS). However, assays types such as FISH and NGS were not provided in this dataset.
5 Conclusions
Patients with advanced ROS1-positive NSCLC who received crizotinib in this real-world population were older, had a higher proportion of smokers, overweight or obese subjects, and had a poorer ECOG performance status than those presented in clinical trials. Despite a greater number of patients with these negative prognostic factors, the outcomes data from this real-world analysis were generally consistent with clinical trial outcomes data and continue to support the clinical benefit of crizotinib in patients with ROS1-positive advanced NSCLC.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. https://doi.org/10.3322/caac.21654.
Surveillance, Epidemiology, and End Results (SEER). Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed June, 2021.
Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin K, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–9. https://doi.org/10.1056/NEJMoa1916623.
Villalobos P, Wistuba II. Lung cancer biomarkers. Hematol Oncol Clin North Am. 2017;31(1):13–29. https://doi.org/10.1016/j.hoc.2016.08.006.
Garber K. ALK, lung cancer, and personalized therapy: portent of the future? J Natl Cancer Inst. 2010;102(10):672–5. https://doi.org/10.1093/jnci/djq184.
Gainor JF, Shaw AT. Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist. 2013;18(7):865–75. https://doi.org/10.1634/theoncologist.2013-0095.
Davies KD, Le AT, Theodoro MF, Skokan MC, Aisner DL, Berge EM, et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res. 2012;18(17):4570–9. https://doi.org/10.1158/1078-0432.CCR-12-0550.
Ou SH. Crizotinib: a novel and first-in-class multitargeted tyrosine kinase inhibitor for the treatment of anaplastic lymphoma kinase rearranged non-small cell lung cancer and beyond. Drug Des Dev Ther. 2011;5:471–85. https://doi.org/10.2147/DDDT.S19045.
Gerson SL, Caimi PF, William BM, Creger RJ. Pharmacology and molecular mechanisms of antineoplastic agents for hematologic malignancies, Chapter 57. In: Hoffman R, Silverstein LE, Weitz JI, et al. (eds.) Hematology: Basic Principles and Practice. 7th ed. Elsevier Inc.; 2018. p. 849–912.
National Comprehensive Cancer Network. Non-Small Cell Lung Cancer Version 2; 2013. https://www.oncomel.org/files/NCCN%20Guidelines%20%20Non%20Small%20Cell%20Lung%20Cancer%202013.pdf. Accessed Sept 22, 2020.
Food and Drug Administration. FDA approves crizotinib capsules. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-crizotinib-capsules. Accessed June 25, 2021.
Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto T, et al. Phase II study of crizotinib in east asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(14):1405–11. https://doi.org/10.1200/JCO.2017.75.5587.
Liu C, Yu H, Chang J, Chen H, Li Y, Zhao W, et al. Crizotinib in Chinese patients with ROS1-rearranged advanced nonsmall-cell lung cancer in routine clinical practice. Target Oncol. 2019;14(3):315–23. https://doi.org/10.1007/s11523-019-00636-6.
Michels S, Gardizi M, Schmalz P, Thurat M, Pereira E, Sebastian M, et al. MA07.05 EUCROSS: a European phase II trial of crizotinib in advanced adenocarcinoma of the lung harboring ROS1 rearrangements—preliminary results. J Thorac Oncol. 2017;12(1):S379–80. https://doi.org/10.1016/j.jtho.2016.11.428.
Landi L, Chiari R, Tiseo M, D’Inca F, Dazzi C, Chella A, et al. Crizotinib in MET-deregulated or ROS1-rearranged pretreated non-small cell lung cancer (METROS): a phase II, prospective, multicenter Two-Arms Trial. Clin Cancer Res. 2019;25(24):7312–9. https://doi.org/10.1158/1078-0432.CCR-19-0994.
Mazieres J, Zalcman G, Crino L, Biondani P, Barlesi F, Filleron T, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33(9):992–9. https://doi.org/10.1200/JCO.2014.58.3302.
Shen L, Lu S. Crizotinib versus pemetrexed-based chemotherapy in patients with advanced ROS1-rearranged non-small cell lung cancer. J Clin Oncol. 2019;37(15 suppl):9101. https://doi.org/10.1200/JCO.2019.37.15_suppl.9101.
Doebele RC, Perez L, Trinh H, Martinec M, Martina R, Riehl T, et al. Time-to-treatment discontinuation (TTD) and real-world progression-free survival (rwPFS) as endpoints for comparative efficacy analysis between entrectinib trial and crizotinib real-world ROS1 fusion-positive (ROS1+) NSCLC patients. J Clin Oncol. 2019;37(15_suppl):9070. https://doi.org/10.1200/JCO.2019.37.15_suppl.9070.
Boyd M, Fulcher N, Annavarapu S, Aguilar K, Frytak J, Robert N, et al. Concordance of death date assessments between the Social Security Death Master File and electronic health records in a US community oncology setting. ISPOR; May 16–20, 2020, Orlando (2020).
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. https://doi.org/10.2307/2670170.
Shaw AT, Riely GJ, Bang YJ, Kim DW, Camidge DR, Solomon BJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30(7):1121–6. https://doi.org/10.1093/annonc/mdz131.
National Comprehensive Cancer Network. Non-small cell lung cancer version 7; 2021. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed Nov 11, 2021.
Wong W, Wu N, Gupta R, Mansfield AS. Utilization trends and factors associated with ROS1 testing among patients with advanced non-small-cell lung cancer in US community practices. Clin Lung Cancer. 2021;22(3):e470–80. https://doi.org/10.1016/j.cllc.2020.06.019.
Walker B, Boyd M, Aguilar K, Davies K, Espirito J, Frytak J, et al. Comparisons of real-world time-to-event end points in oncology research. JCO Clin Cancer Inform. 2021;5:45–6. https://doi.org/10.1200/cci.20.00125.
Waterhouse DM, Tseng WY, Espirito JL, Robert NJ. Understanding contemporary molecular biomarker testing rates and trends for metastatic NSCLC among community oncologists. Clin Lung Cancer. 2021. https://doi.org/10.1016/j.cllc.2021.05.00.
Lim C, Tsao MS, Le LW, Shepherd FA, Feld R, Burkes RL, et al. Biomarker testing and time to treatment decision in patients with advanced nonsmall-cell lung cancer. Ann Oncol. 2015;26(7):1415–21. https://doi.org/10.1093/annonc/mdv208.
American Cancer Society. Improving access to biomarker testing. Advancing precision medicine in cancer care. https://www.fightcancer.org/sites/default/files/Improving%20Access%20to%20Biomarker%20Testing_FINAL.pdf. Accessed May 3, 2021.
Acknowledgements
The research described in this article was presented at the World Conference on Lung Cancer (WCLC), 28-31 January 2021, Singapore. This study was sponsored by Pfizer, Inc and conducted by Ontada. The authors would like to acknowledge Lisa Kaspin-Powell, PhD, ELS, an employee of Ontada, for editorial support, which was funded by Pfizer, Inc. The authors would like to acknowledge Jie Zhou, PhD, an employee of Ontada at the time of data analysis, for editorial support, which was funded by Pfizer, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was sponsored by Pfizer, Inc.
Authorship disclosures
Sneha Sura is an employee of Ontada, which was a paid consultant to Pfizer in connection with the development of this article. Janet Espirito is an employee of Ontada, which was a paid consultant to Pfizer in connection with the development of this article. David Waterhouse is an employee of OHC. OHC is an affiliate of US Oncology/McKesson McKesson, which was a paid consultant to Pfizer in connection with the development of this article.
Conflict of interest
David Waterhouse has an advisory role/consulting from Bristol Myers Squibb, AZTherapies, AbbVie, Amgen, McGivney Global Advisors, Janssen Oncology, Seattle Genetics, Jazz Pharmaceuticals, Exelixis, Eisai, EMD Serono, Merck, Pfizer, Mirati Therapeutics, and Regeneron/Sanofi, speakers’ bureau from Bristol Myers Squibb, Janssen Oncology, Merck, AstraZeneca, and travel, accommodation and expenses from Bristol Myers Squibb. Laura Iadeluca is an employee of Pfizer, Inc. and owns stock in Pfizer, Inc. Sneha Sura is an employee of Ontada. Keith Wilner is an employee of Pfizer, Inc. and owns stock in Pfizer, Inc. Birol Emir is an employee of Pfizer, Inc. and owns stock in Pfizer, Inc. Janet Espirito is an employee of Ontada and owns stock in McKesson, which received consulting fees in connection with this study from Pfizer, Inc. Lauren Bartolome is an employee of Pfizer, Inc. and owns stock in Pfizer, Inc. Stan Krulewicz is an employee of Pfizer, Inc. and owns stock in Pfizer, Inc.
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals
Not applicable.
Ethics approval
The study protocol received an exception and waiver of informed consent from the US Oncology Institutional Review Board.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Code availability
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Waterhouse, D., Iadeluca, L., Sura, S. et al. Real-World Outcomes Among Crizotinib-Treated Patients with ROS1-Positive Advanced Non-Small-Cell Lung Cancer: A Community Oncology-Based Observational Study. Targ Oncol 17, 25–33 (2022). https://doi.org/10.1007/s11523-021-00860-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-021-00860-z