Abstract
Introduction
Ovarian cancer (OC) is one of the leading causes of cancer mortality among women in the United States. With the approval of first-line maintenance therapies, patients with OC experienced prolonged first-line progression-free survival. While the literature addresses some costs associated with OC, further research is needed on the costs of progression that are potentially deferred or prevented by early maintenance. The objective of this study was to capture the health care resource utilization and costs of patients with advanced OC who never received poly(ADP ribose) polymerase (PARP) inhibitor maintenance.
Methods
We conducted a descriptive retrospective analysis of treatment patterns and the consequences of progression through several lines of therapy (LOTs) in patients with OC, using claims from commercial and Medicare Advantage health plan members in the United States from the Optum Research Database between January 1, 2010, and April 30, 2019. Patients were required to have an index OC diagnosis (≥ 2 non-diagnostic claims). We examined up to 4 LOTs and the time between treatments.
Results
A total of 5498 women met the eligibility criteria. As the number of LOTs increased, the median duration of each line decreased from 137 days in LOT1 to 94 days in LOT4, and the time between lines also decreased from 245 to 0 days. Ambulatory care visits were a major driver of health care resource utilization, with a median of about 6 monthly visits during active treatment. The mean total monthly health care costs for patients with at least 2 LOTs were US$8588 (SD: $8533) before LOT2 and increased to $15,358 (SD: $21,460) during or after LOT2.
Conclusions
Prolonging progression-free survival after first-line treatment in patients with OC may provide the opportunity to delay or prevent later treatment, the financial toxicity felt by patients, and the economic burden to the health care system associated with progression.
Plain Language Summary
Ovarian cancer is a complex disease in which > 70% of patients are diagnosed with advanced disease, and one of the leading causes of cancer mortality among women in the United States. A variety of maintenance therapy options, including bevacizumab, PARP inhibitors, and PARP plus bevacizumab combination therapies, have demonstrated improvements in progression-free survival. By delaying disease progression after completion of first-line therapy, a simultaneous decrease in post-progression health care costs may be seen. The objective of this study was to capture the health care resource utilization and costs of patients with advanced ovarian cancer who did not receive a PARP inhibitor at any time in their treatment
In patients never receiving a PARP inhibitor, this study documented substantial health care resource usage and costs associated with progression beyond the first line of treatment (surgery and/or chemotherapy) in ovarian cancer. These were largely driven by the number of ambulatory care visits. When these visits are combined with emergency department visits and inpatient stays, high costs are incurred by both patients and third-party payers
Prolonging progression-free survival after first-line treatment in patients with ovarian cancer may delay or prevent the need for later treatment, the financial burden felt by patients, and the economic burden to the health care system associated with subsequent disease progressions
Similar content being viewed by others
Emerging therapies have demonstrated the ability to delay first progression in advanced ovarian cancer |
There has been limited research on the health care resource utilization and costs associated with the long-term treatment of ovarian cancer |
Monthly treatment costs for ovarian cancer increase substantially after first progression, largely driven by the number of ambulatory care visits |
Prolonging the time to first recurrence in ovarian cancer can delay or prevent the high costs associated with progression |
Introduction
Ovarian cancer is a complex disease and one of the leading causes of cancer mortality among women in the United States. Epithelial ovarian carcinomas, which include ovarian, fallopian tube, and primary peritoneal carcinomas, comprise approximately 90% of ovarian cancers [1, 2]. In 2021, approximately 24,410 new cases of ovarian cancer with 13,770 deaths will have occurred [3]. Notably, most women (> 70%) are diagnosed with advanced stages of the disease [1, 4, 5].
Treatment of advanced ovarian cancer generally consists of cytoreductive surgery and platinum- and taxane-based chemotherapy. Approximately 80% of patients’ tumors are initially responsive to this treatment [6]. Unfortunately, the majority of women with advanced-stage ovarian cancer will relapse and require additional treatment. Due to the high rate of recurrence, which is typically metastatic, 5-year survival rates are 49% [3].
The management of recurrent ovarian cancer is largely directed by the time elapsed between the completion of platinum-based treatment and the detection of relapse, known as the platinum-free interval (PFI) [7, 8]. Those patients with a PFI of 6 months or longer are considered platinum-sensitive, and are also considered to have a much better prognosis than those that have a recurrence sooner. Responses to subsequent platinum-based therapy range between 30 and 90% [9,10,11]. Patients with a PFI of less than 6 months or who progress on upfront platinum therapy or within 1 month of completing therapy are considered platinum-resistant or platinum-refractory, respectively. In this population, response rates to subsequent therapies are typically < 15% [12].
When treating advanced and recurrent ovarian cancer, a patient’s somatic and genomic profiles are critical, specifically BRCA and markers of genomic instability. Patients harboring these mutations and phenotypic changes are considered to have homologous recombination-deficient (HRD) tumors. These tumors are unable to repair double-strand DNA breaks and are particularly sensitive to poly(ADP ribose) polymerase (PARP) inhibitors, which inhibit DNA repair of single-strand breaks. This dual inhibition results in synthetic lethality [13].
Several therapies have been developed to help delay the time to recurrence in patients with advanced ovarian cancer after surgery and chemotherapy. Two PARP inhibitors are currently approved for use as first-line maintenance therapy in patients who have a complete or partial response to first-line platinum chemotherapy. Olaparib is approved as first-line maintenance monotherapy for patients with a deleterious or suspected deleterious germline or somatic BRCA mutation or in combination with bevacizumab for patients with a positive HRD status (determined to have a BRCA mutation or genomic instability on a companion diagnostic) [14]. Niraparib is approved for first-line maintenance regardless of biomarker status [15]. In addition to use in first-line maintenance, several PARP inhibitors are indicated for use in a relapsed setting [14,15,16]. Bevacizumab monotherapy has also demonstrated efficacy as another option to delay progression [17].
Although a significant body of literature has addressed the costs of maintenance treatment and the cost effectiveness of various pharmacologic and surgical treatments in ovarian cancer, some costs have not been captured in these studies. There has been a relative paucity of literature on initial care costs, and little has been published on the costs related to the progression of ovarian cancer. Urban et al. reported that the initial US cost of surgery and adjuvant chemotherapy was US$83,915 per patient [18]. Another US study indicated that the median cost of primary debulking surgery was $89,228 and neoadjuvant chemotherapy was $92,770, with patients facing median adjusted out-of-pocket costs of $2977 and $2519 [19]. Although these studies provide some information on the costs of ovarian cancer treatment, they do not include detailed health care resource utilization (HCRU) information, such as the number of ambulatory care visits, and do not provide information on the costs incurred after the first line of therapy (LOT1).
Because maintenance therapy options such as PARP inhibitors or bevacizumab can, in many cases, delay progression for a significant period of time even after patients discontinue therapy, further research is needed on the costs of progression that are potentially deferred or even prevented. In addition, to fully understand the cost of ovarian cancer treatment, more detailed information is needed on the costs of first-line treatments before a patient experiences recurrence. Beyond the clinical and emotional impact of disease relapse and associated treatment, these costs contribute to the financial toxicity felt by many patients with cancer. In a recent study of gynecologic patients (n = 121; mean age, 59 years), over half reported financial distress. Those with financial distress, measured on a validated questionnaire, were significantly more likely to report mental hardship, lost wages, the inability to pay bills on time, and the need to borrow money. Patients aged younger than 65 years and with an income of less than $40,000 had increased risk of financial distress [20].
The goal of this study is to understand the potential burden and costs that may be offset by delaying progression through capturing the HCRU and costs of patients with commercial and Medicare Advantage health insurance who had advanced ovarian cancer and did not receive a PARP inhibitor at any point during their treatment.
Methods
Study Design
We conducted a descriptive retrospective analysis of treatment patterns and the consequences of progression (treatment beyond LOT1) through several LOTs in patients with ovarian cancer using claims from commercial and Medicare Advantage health plan members in the United States from the Optum Research Database (ORD) between January 1, 2010, and April 30, 2019 (identification period).
Data Sources
The ORD, Optum’s proprietary administrative claims research database, is one of the largest and most complete insurance claims research databases in the United States. The ORD is fully de-identified and Health Insurance Portability and Accountability Act (HIPAA)-compliant, and comprises medical and pharmacy claims data (including linked enrollment) from 1993 to the present for more than 84 million patients [21]. Features include eligibility control (complete member information with enrollment dates), medical and pharmacy claims (delivers insight into all health care services), and cost information (measures resource utilization plus paid amounts that reflect the intensity of the service provided). In 2016, approximately 19% of the US commercially enrolled population, plus 17% of the Medicare Advantage and 23% of the Medicare prescription drug plan population, were represented in the ORD.
Medical and pharmacy claims data have been available for approximately 8.9 million Medicare Part C (Medicare Advantage) enrollees since 2006. Beginning in 2006, complete medical and pharmacy information became available for Medicare enrollees with medical and Part D coverage. Costs represent paid claims to providers including patient copays. Pharmacy claims contain sufficient information to trace patients’ pharmacy expenditures through the multiple phases of the Part D plans, which are structured to change over time, with a gradual elimination of the gap in coverage.
Claims for pharmacy services were typically submitted electronically by the pharmacy at the time prescriptions were filled. The claims history is a profile of all outpatient prescription pharmacy services provided and covered by the health plan. Pharmacy claims data include National Drug Code (NDC), fill date, quantity supplied, drug strength, days of supply, financial information, and de-identified patient and prescriber codes, allowing for longitudinal tracking of medication refill patterns and changes in medications. Pharmacy claims were typically added to the research database within 6 weeks of dispensing.
Data on the occurrence of death were derived from the Social Security Administration Death Master files, as well as from records with codes indicating death within hospital discharge status. By including external mortality data in this study, it was possible to determine the timing of deaths that were not otherwise captured in the claims. After subjects were linked, these data were used to inform sample selection. Subjects who might otherwise be removed from the study owing to insufficient continuous enrollment may be included if they were enrolled until death. If included, mortality data can also be used to modify outcomes and other study endpoints.
Study Population
Patients were required to have an index ovarian cancer diagnosis [≥ 2 non-diagnostic claims for ovarian cancer (International Classification of Diseases, Ninth Revision, Clinical Modification: 183.0*, 183.2*; International Classification of Diseases, Tenth Revision, Clinical Modification: C56*, C570*) ≥ 30 days apart during the identification period, January 1, 2010, through April 30, 2019] and to have received systemic therapy that included both National Comprehensive Cancer Network-recommended agents as well as other systemic chemotherapies between the index date and the end of the identification period [22, 23].
Diagnostic claims are those claim lines that have codes dealing with laboratory tests, various imaging services (magnetic resonance imaging, X-ray, etc.), or other processes that fall under the realm of “testing”. These claims were not used for identification purposes because the diagnosis on the claim may not represent a definitive diagnosis, but rather a screening for a disease. Patients were required to have at least 6 months of baseline claims data before the index date and data from at least 6 months after (including the index date) or until the first claim for chemotherapy on or after the index date. They were further required to be aged 18 years or older, with no evidence of receiving a PARP inhibitor at any time during the study period. Patients with evidence of chemotherapy or ovarian cancer in the baseline period were excluded.
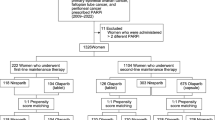
Patients were followed for a variable amount of time after the index date. We examined up to 4 lines of active therapy and also subsequent maintenance LOTs. Since claims data do not contain actual LOTs, we applied an algorithm that used infusion date (for infused medications) and fill date/day supply (oral medications) to identify active and maintenance LOTs. Patients with any record of receiving a PARP inhibitor were excluded. The study team then met to review the initial study output to determine if revisions needed to be made to the LOT algorithm and the active versus maintenance classification (Fig. 1).
Line of Therapy Algorithm
An algorithm was used to identify the start and end of the LOT. The date the patient first received systemic chemotherapy was the start of the first LOT. All anticancer agents filled/infused within the first 30 days following the start of the first LOT defined the regimen. The end was identified as the earliest of any of the following: initiation of a new agent not included in the regimen; discontinuation of all agents in the regimen; death; disenrollment; or end of the study period. If an episode contained bevacizumab plus other medications and all medications besides maintenance were discontinued, then the end of the LOT with monotherapy bevacizumab was classified as maintenance treatment. Maintenance lines were grouped with the preceding active LOT.
Statistical Analysis
All study variables were analyzed descriptively. The number of patients and percentages were provided for categorical variables. Medians with interquartile ranges (IQRs) were used to describe demographics, clinical characteristics, and health care resource use. Means with standard deviation were used to describe costs in order to better inform payers and policymakers about the expected costs of treatment. All costs were adjusted to 2019 dollars. Descriptive techniques that account for length of observation time [e.g., per-patient-per-month (PPPM) amounts] were used where appropriate. No adjustment for multiple comparisons was made.
Protection of Human Subjects
Data were de-identified in compliance with the HIPAA Privacy Rule when disclosed for this research. The appropriate review was performed for the use of discharge status (death status and month/year of death) from hospital discharge claims. The review request was submitted because the study design supports the use of the mortality information of the patients. The full date of death was not used in the study data; rather, date of death was established as the 15th day of the month in which the patient died (only month and year of death were disclosed).
Compliance with Ethics Guidelines
This article does not contain data from any studies with human participants or animals performed by the authors.
Results
Patient Characteristics
Patient characteristics and attrition are contained in Fig. 2. For all patients not receiving a PARP inhibitor (n = 5498), the median (IQR) age was 64 years (55, 72); 56.8% of patients had commercial insurance and 43.2% had Medicare (Table 1) [22, 23]. During the study period, 1652 patients received second-line therapy (LOT2), 884 received third-line therapy (LOT3), and 447 received fourth-line therapy (LOT4; Table 2). Median time in or between LOT decreased between LOT1 and LOT4. Patients spent a median (IQR) of 137 (95.0–167.0), 119 (73.0–178.0), 103 (62.0–164.5), and 94 (58.0–157.0) days in LOT1 through LOT4, respectively. Median time between LOT1 and LOT2, LOT2 and LOT3, and LOT3 and LOT4 decreased and were 245 (137.0–439.0), 10 (0.0–137.0), and 0 (0.0–54.0) days, respectively. All patients received systemic therapy per the inclusion criteria, and the majority of patients (58.8%) had at least one surgery during the follow-up period post-ovarian cancer diagnosis (Table 1).
Health Care Resource Utilization Prior to First Progression
Nearly all patients (99.9%) had an ambulatory care visit prior to progression. The monthly median number of visits was 6.5 from diagnosis to start of LOT1, 6.4 during LOT1, and 3.6 between LOT1 and LOT2 (Table 3); these visits included those for chemotherapy or drug administration, monitoring, and routine care, as well as visits to routine providers including oncologists. About one in three patients had an emergency department visit before, during, or after LOT1 (30.9%, 34.8%, and 37.2%, respectively). A large percentage (72.6%) had an inpatient stay prior to starting therapy, likely due to surgery, but this declined during and after LOT1 (25.7% and 34.5%).
Health Care Costs Prior to First Progression
Mean total health care costs during the initial phases of treatment and surveillance were similar: $45,469 from diagnosis to start of LOT1, $49,184 during LOT1, and $47,348 between LOT1 and LOT2 (Table 4) [24]. Mean PPPM health care costs prior to progression varied during this same time period: $26,172 from diagnosis to start of LOT1, $10,820 during LOT1, and $5011 between LOT1 and LOT2.
Health Care Resource Utilization After First Progression
Nearly all patients had at least one ambulatory care visit (99.7–99.8%), and median all-cause ambulatory visits after first progression were similar during each LOT (24.0, 20.0, and 19.0 during LOT2, LOT3, and LOT4, respectively). This is equivalent to a median of 5.9, 6.1, and 6.0 visits per month. Median ambulatory care visits were infrequent between LOTs (Table 5). For patients with 4 LOTs, median total ambulatory visits were 97.0 after first progression. Similar to before progression, about one-third of patients experienced an emergency department visit during each additional LOT (38.8%, 39.1%, and 38.0%). Rates for inpatient stays were similar between LOTs (29.2%, 32.7%, and 35.4%). For patients who had 4 LOTs, 69.6% had at least one emergency department visit and 59.5% had at least one inpatient stay after progression.
Health Care Costs After First Progression
Mean total health care costs for each additional LOT ranged from $53,158 to $60,217, with an additional $12,899 to $13,326 between lines. This is equivalent to a monthly per patient cost of between $15,417 and $20,654 during active treatment and $2928 to $3080 between treatments. Mean total health care costs after first progression for patients with 4 LOTs were $186,713 (Table 6) [24].
Monthly Total Pre- and Post-LOT2 Progression for Patients with at Least 2 Lines of Therapy
Importantly, the mean total PPPM health care cost for patients with at least 2 LOTs was $8588 (SD: $8533) before LOT2 which increased to $15,358 (SD: $21,460) (Table 7) during or after LOT2 [24].
Discussion
Summary of Findings
This study documented substantial HCRU and costs associated with progression beyond LOT1 in ovarian cancer, which were largely driven by the number of ambulatory care visits. These visits, combined with the burden of emergency department and inpatient stays, present a large impact to patients’ lives and high costs to both patients and third-party payers. The average cost of cancer care reimbursed by payers and patient copays during each active line of therapy was substantial, and increased as patients progressed in their treatments. Yue et al. identified annual costs of $13,566 for patients with ovarian cancer [25]. While observed costs in this study were higher, the difference may be due to the study sample. Patients included in this study were actively being treated for ovarian cancer, and may have progressed through multiple LOTs. Patients with neoadjuvant therapy only were excluded from the study. In addition, costs after first LOT were only examined in the subset of patients who progressed to a second LOT.
In addition to the burden on the healthcare system, high costs impact patients as this study includes copays and coinsurance borne by individuals. In a review of Washington state cancer patients, cancer patients were 2.5 times more likely to file for bankruptcy after a cancer diagnosis compared to those without a cancer diagnosis [26], and filing of bankruptcy was associated with increased mortality among evaluated cancer types (hazard ratio 1.79; 95% confidence interval, 1.64–1.96) [27].
Prolonging progression-free survival (PFS) in patients with ovarian cancer has the potential to delay cancer-directed treatment and the economic burden and financial toxicity associated with progression. Liang et al. found that proactive intervention early in the treatment course reduced financial stress in patients [20]. Our results indicate that there is the least amount of variation in costs during LOT1. The greatest variability in costs was between LOT1 and LOT2 when most patients, though not all, often progress.
This variability in costs may be due to clear and standardized treatment in the upfront management of patients with ovarian cancer. For patients that recur, treatment is less standardized, as there is no one evidence-based best treatment option. In a meta-analysis of three phase 3 randomized control trials, median PFSs after first, second, third, fourth, and fifth relapse were 10.2, 6.4, 5.6, 4.4, and 4.1 months, respectively (306, 192, 168, 132, and 123 days) [28]. Along with the decreasing PFS, variability in treatment increased, and at least 20% of platinum-resistant and 46% of platinum-sensitive patients did not receive a “state-of-the-art” therapy. While our study was not designed to assess PFS, the median time between LOT1 and LOT2 (or first relapse), LOT2 and LOT3, and LOT3 and LOT4 similarly decreased, and was 245, 10, and 0 days, respectively, not including the time on treatment. We also observed a 58.8% rate of surgery during the study follow-up period, which likely accounts for the high rate of inpatient stays early in the treatment pathway. This rate is in line with the 60% surgery rate reported by physician surveys for patients with Stage IV disease, but lower than the 81%–91% rate for earlier stages of disease [29], and may reflect that patients undergoing upfront debulking prior to diagnosis were not entirely captured by our algorithm.
Methods to Minimize Bias
We included methods to minimize bias. For studies involving retrospective health care data, the most common sources of bias included missing data, incorrect classification of diagnoses, procedures, and medications, and unobservable differences in patient characteristics. Missing data could introduce bias into the study if the data were systematically missing for selected samples. However, health care claims were generally required to contain the relevant pieces of information for the claim to be processed. All medical claims were required to have a diagnosis code, and all pharmacy claims had a corresponding NDC code. Lastly, we anticipated that < 1% of the sample would be excluded because of missing demographic information.
Another possible source of bias was the incorrect classification of diagnoses, procedures, and medications. To limit this bias, the study team determined the study code list and study algorithm in consultation with the team’s medical directors. Although it was possible that incorrect codes were used, the team did not expect that the rate of inaccurate coding would be high, based on a review of billing audits, or that it would be distributed systematically among only one cohort of the patients in this study.
Unobservable differences in patient characteristics could have influenced findings. The goal of this study was to examine the real-world treatment patterns associated with ovarian cancer. Therefore, although there were unobservable differences, these were expected to be reflective of the populations outside the controlled setting of a clinical trial. This study used all patients who were diagnosed with ovarian cancer in the ORD, which used data from a large US health care database that was geographically diverse.
Strengths and Limitations
The study population represented both commercial insurance and Medicare Advantage patients, and had a broad geographical distribution in the United States. In addition, patient data were available from all health care providers for resource utilization and costs, so the full impact of care for ovarian cancer was captured. However, as with all retrospective databases, given the lack of randomization and subsequent bias, there were issues with confounding, misclassification, selection, generalizability, and random error; thus, the results should be viewed with a certain amount of caution.
Claims data are extremely valuable for the effective examination of treatment patterns, outcomes such as time to first subsequent treatment or death, HCRU, and costs. However, all claims databases have certain inherent limitations because the claims are collected for the purpose of payment and not research: (1) a claim for a filled prescription is not an indication that the medication was consumed or taken as prescribed; (2) physician-provided samples, samples taken as part of a clinical trial, or over-the-counter medications will not be observed in claims data; (3) the presence of a diagnosis code on a medical claim does not confirm the presence of disease, as the diagnosis code may be incorrectly coded or included as rule-out criteria rather than actual disease; (4) other costs borne by the patients such as transportation, lost work productivity, etc., are not included; and (5) while costs were incurred by patients undergoing treatment for ovarian cancer, it is possible that not all costs were for the treatment of ovarian cancer. Patients with comorbidities will have incurred costs independent of their cancer.
In addition, studies of patients with ovarian cancer may be limited by several factors. The ORD is updated monthly and subject to a medical claim lag of approximately 6 months. Therefore, this study was able to use data through October 2019. The index date was also set at the diagnosis of ovarian cancer and may not capture resource use, such as surgery, that occurred before a formal diagnosis.
Patients receiving PARP inhibitors were excluded to better understand the impact of the natural course of disease without maintenance treatment. Bevacizumab was not excluded due to the fact that it is utilized concurrently with other agents, as a monotherapy for treatment, and as maintenance. Because of this triple use, it is difficult in the claims data to separate differences between treatment and maintenance; therefore, some patients may have received bevacizumab as a maintenance option.
Because LOT was not a measure that is captured in the claims data, we implemented algorithms based on the day that patients received chemotherapies. To ensure that algorithms for active treatment lines and maintenance treatment lines were accurate, the team elicited feedback from the study medical directors and also examined empirical data and de-identified patient examples. Although disease stage was not included in the claims assessment, based on the percentage with a metastatic diagnosis and systemic therapy, we believe most patients had advanced disease.
Conclusions
This study documented extensive HCRU and costs associated with progression beyond LOT1 in ovarian cancer, which were largely driven by the high number of ambulatory care visits during therapy. Prolonging PFS after first-line treatment in patients with ovarian cancer has the potential to delay treatment and to increase the economic burden to patients and the health care system.
References
West K, Borley J. Ovarian, fallopian tube and primary peritoneal cancer: an overview. Obstet Gynaecol Reprod Med. 2020;30(12):380–6.
Kim J, Park EY, Kim O, et al. Cell origins of high-grade serous ovarian cancer. Cancers. 2018;10:433.
Cancer Stat Facts: Ovarian Cancer. National Cancer Institute, Surveillance, Epidemiology, and End Results Program. https://seer.cancer.gov/statfacts/html/ovary.html. Accessed 13 May 2021
Doubeni CA, Doubeni ARB, Myers AE. Diagnosis and management of ovarian cancer. Am Fam Phys. 2016;93(11):937–44.
American Cancer Society. Cancer Facts & Figures 2018. Special Section: Ovarian Cancer. Atlanta: American Cancer Society; 2018.
Sato S, Itamochi H. Neoadjuvant chemotherapy in advanced ovarian cancer: latest results and place in therapy. Ther Adv Med Oncol. 2014;6(6):293–304.
Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69:280–304.
Dockery LE, Rubenstein AR, Ding K, et al. Extending the platinum-free interval: the impact of omitting 2nd line platinum chemotherapy in intermediate platinum-sensitive ovarian cancer. Gynecol Oncol. 2019;155(2):201–6.
Rose PG, Blessing JA, Mayer AR, Homesley HD. Prolonged oral etoposide as second-line therapy for platinum-resistant and platinum-sensitive ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 1998;16(2):405–10.
Balbi G, Di Prisco L, Musone R, et al. Second-line with paclitaxel and carboplatin for recurrent disease following first paclitaxel and platinum compounds in ovarian carcinoma. Eur J Gynaecol Oncol. 2002;23(4):347–9.
Hoekstra AV, Hurteau JA, Kirschner CV, Rodriguez GC. The combination of monthly carboplatin and weekly paclitaxel is highly active for the treatment of recurrent ovarian cancer. Gynecol Oncol. 2009;115(3):377–81.
Davis D, Tinker AV, Friedlander M. “Platinum resistant” ovarian cancer: what is it, who to treat and how to measure benefit? Rev Gynecol Oncol. 2014;133(3):624–31.
Ma J, Setton J, Lee NY, Riaz N, Powell SN. The therapeutic significance of mutational signatures from DNA repair deficiency in cancer. Rev Nat Commun. 2018;9(1):3292.
Olaparib. Prescribing information. AstraZeneca Pharmaceuticals LP; 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208558s014lbl.pdf. Accessed 16 Feb 2021
Niraparib. Prescribing information. Tesaro, Inc. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208447lbl.pdf. Accessed 16 Feb 2021
Rucaparib. Prescribing information. Clovis Oncology, Inc. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/209115s000lbl.pdf. Accessed 16 Feb 2021
Bevacizumab. Prescribing information. Genentech, Inc. 2021. https://www.gene.com/download/pdf/avastin_prescribing.pdf. Accessed 16 Feb 2021
Urban R, He H, Alfonso-Cristancho R, Hardesty M, Goff B. The cost of initial care for Medicare patients with advanced ovarian cancer. J Natl Compr Canc Netw. 2016;14(4):429–37.
Suidan R, He W, Sun C, et al. Total and out-of-pocket costs of different primary management strategies in ovarian cancer. Am J Obstet Gynecol. 2019;221(2):136.e1-136.e9.
Liang MI, Pisu M, Summerlin SS, et al. Extensive financial hardship among gynecologic cancer patients starting a new line of therapy. Gynecol Oncol. 2020;156(2):271–7.
Health Economics and Outcomes Research (HEOR). OPTUM. https://www.optum.com/business/solutions/life-sciences/answer-research/heor.html. Accessed 3 Feb 2021
Clinical Classification Software (CCS) for ICD-9-CM. Agency for Healthcare Research and Quality. March 2017. www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed 17 Dec 2021
Clinical Classification Software (CCS) for ICD-10-PCS (beta version). Agency for Healthcare Research and Quality. November 2019. www.hcup-us.ahrq.gov/toolssoftware/ccs10/ccs10.jsp. Accessed 15 Feb 2021
US Department of Labor, Bureau of Labor Statistics. Chained CPI for all urban consumers, US city average (C-CPI-U). Medical Care. Series ID: SUUR0000SAM. 2012. http://data.bls.gov/cgi-bin/surveymost?su. Accessed 16 Feb 2021
Yue X, Pruemer JM, Hincapie AL, Almalki ZS, Guo JJ. Economic burden and treatment patterns of gynecologic cancers in the United States: evidence from the Medical Expenditure Panel Survey 2007–2014. J Gynecol Oncol. 2020;31(4):e52. https://doi.org/10.3802/jgo.2020.31.e52.
Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood). 2013;32(6):1143–52.
Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34(9):980–6.
Hanker LC, Loibl S, Burchardi N, AGO and GINECO study group, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23(10):2605–12. https://doi.org/10.1093/annonc/mds203.
CancerMPact®. Cerner Enviza. https://www.cernerenviza.com/syndicated-insights-and-reports-for-life-sciences/cancermpact. Accessed 3 Mar 2022
Acknowledgements
Funding
This study was funded by AstraZeneca and is part of an alliance between AstraZeneca and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. AstraZeneca funded the Rapid Service and Open Access fees.
Medical Writing Assistance
The authors wish to thank Leonard Lionnet, PhD, CMPP, of Kay Square Scientific for providing medical writing assistance, which was supported by AstraZeneca and is part of an alliance between AstraZeneca and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Authorship
All authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All named authors contributed to interpretation of data, drafting of the manuscript, and critical revision of the manuscript. Daniel Simmons, Benjamin Chastek, Scott H. Bunner, and Kimmie McLaurin contributed to the conception and design of the study. Benjamin Chastek and Scott H. Bunner were responsible for data acquisition and statistical analysis.
Disclosures
Daniel Simmons and Kimmie McLaurin are employees of AstraZeneca. Benjamin Chastek and Scott H. Bunner are employees of Optum, which was hired by AstraZeneca to conduct the study. Adam C. ElNaggar reports research support from AstraZeneca, Caris Life Sciences, and Tesaro/GSK, as well as consulting fees from AbbVie, AstraZeneca, Clovis Oncology, Leap Therapeutics, and Merck during the conduct of the study. Since completion of the study, affiliation for Adam C. ElNaggar has changed to Natera, Inc., San Carlos, CA, USA. Stephanie V. Blank has nothing to disclose.
Compliance With Ethics Guidelines
This article does not contain data from any studies with human participants or animals performed by the authors.
Data Availability
Data sharing is not applicable to this article as no new datasets beyond the Optum Research Database were generated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Simmons, D., Blank, S.V., ElNaggar, A.C. et al. Health Care Resource Utilization and Costs Associated with Disease Progression in Ovarian Cancer. Adv Ther 39, 2544–2561 (2022). https://doi.org/10.1007/s12325-022-02086-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02086-5