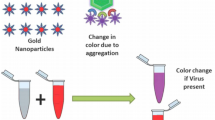
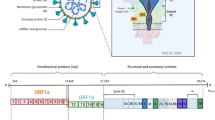
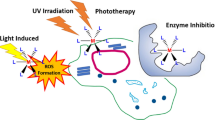
Abstract
The outbreak of SARS-CoV-2 has resulted in an unprecedented and greatest global health crisis in the present century affecting more than 220 countries with 3.7 million deaths and 173.5 million individual infections till now. This pandemic has had an enormous impact on global healthcare, economy, and society, which has prompted extensive research on exploring the biology of SARS-CoV-2 and the discovery of new drugs for COVID-19. The lack of effective antiviral drugs for COVID-19 has initiated the effort to repurpose selected FDA-approved antiviral drugs for the treatment of COVID-19 along with plasma therapy. Vaccination has proven to be the effective prevention strategy against the SARS-CoV-2 virus, although mutations in the SARS-CoV-2 virus have become the major concern due to the decreasing effectiveness of the vaccines Therefore, an effective cure for COVID-19 is still an elusive goal. Transition metal complexes by a broad spectrum of formal charge and oxidation states, wide range of coordination number and geometry, tunable kinetic, thermodynamic, and redox properties, diverse reaction pathways have emerged as the alternative and viable tools in the medicinal domain from therapeutics to diagnostics. Several transition metal complexes proved their efficacy against various types of viruses and recent advances on the potent transition metal complexes or nanoconjugates are reviewed in this chapter. The present chapter also aims to discuss the perspectives on the potential utility of transition metal complexes or the nanoconjugates against SARS-CoV-2.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395:565–574
https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang NB, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W (2020) A novel coronavirus from patients with pneumonia in China. Engl J Med 382:727–733
Baig AM, Khaleeq A, Ali U, Syeda H (2020) Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11:995–998
Aminnejad R, Shafiee H (2020) Is regional anesthesia safe enough in suspected or confirmed COVID-19 patients? ACS Chem Neurosci 11:1371–1371
Xiong C, Jiang L, Chen Y, Jiang Q (2020) Evolution and variation of 2019-novel coronavirus. bioRxiv. https://doi.org/10.1101/2020.01.30.926477
Guerra S (2020) Oral mucosal lesions in a COVID-19 patient: New signs or secondary manifestations? Int J Infect Dis 97:326–328
https://en.wikipedia.org/wiki/National_responses_to_the_COVID-19_pandemic
Godbole T (2020) Domestic violence rises amid coronavirus lockdowns in Asia 11 April, 2020
Mental health and psychosocial considerations during the COVID-19 outbreak. World Health Organization. 18 March 2020
https://en.m.wikipedia.org/wiki/Social_impact_of_the_COVID-19_pendemic
Opatz T, Senn-Bilfinger J, Richert C (2020) Thoughts on what chemists can contribute to fighting SARS-CoV-2—a short note on hand sanitizers, drug candidates and outreach. Angew Chem Int Ed 59:9236–9240
https://www.weforum.org/agenda/2020/02/coronavirus-economic-effects-global-economy-trade-travel/
Leng Z, Zhu R, Hou W, Feng Y, Liu H, Jin R, Jin K, Zhao RC (2020) Transplantation of ACE2—mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis 11:216–228
https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
Corman VM, Muth D, Niemeyer D, Drosten C (2018) Hosts and sources of endemic human coronaviruses. Adv Virus Res 100:163–188
Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, Wit E, Munster VJ (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382:1564–1567
Shalev D, Shapiro PA, Psychiatry E (2020) Epidemic psychiatry: the opportunities and challenges of COVID-19. Gen Hosp Psychiatry 64:68–71
Encinar JA, Menendez JA (2020) Potential drugs targeting early innate immune evasion of SARS-coronavirus 2 via 2′-O-methylation of viral RNA. Viruses 12:525
Tang X, Wu C, Li X, Song Y, Yao X, Wu X, Duan Y, Zhang H, Wang Y, Qian Z, Cui J, Lu J (2020) On the origin and continuing evolution of SARS-CoV-2. Natl Sci Rev 7:1012–1023
Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, Myers R, Campbell C, Amirthalingam G, Edmunds M, Zambon M, Brown K, Hopkins S, Chand M, Ramsay M (2021) Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. https://doi.org/10.1101/2021.05.22.21257658. Zhou D, Dejnirattisai W, Supasa P, Liu C, Mentzer AJ, Ginn HM, Zhao Y, Duyvesteyn HME, Tuekprakhon A, Nutalai R, Wang B, Paesen GC, Lopez-Camacho C, Slon-Campos J, Hallis B, Coombes N, Bewley K, Charlton S, Walter TS, Skelly D, Lumley SF, Dold C, Levin R, Dong T, Pollard AJ, Knight JC, Crook D, Lambe T, Clutterbuck E, Bibi S, Flaxman A, Bittaye M, Belij-Rammerstorfer S, Gilbert S, James W, Carroll MW, Klenerman P, Barne E, Dunachie SJ, Fry EE, Mongkolsapaya J, Ren J, Stuart DI, Screaton GR (2021) Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 198:2348–2361
Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F (2020) Structural basis of receptor recognition by SARS-CoV-2. Nature 581:221–224. Chang EL, Simmers C, Knight DA (2010) Cobalt complexes as antiviral and antibacterial agents. Pharmaceuticals 3:1711–1728
Musib D, Raza MK, Kundu S, Roy M (2018) Modulating in vitro photodynamic activities of copper(II) complexes. Eur J Inorg Chem 2018:2011–2018
Lu R, Zhao X, Li J (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395:565–574
Walls AC, Park Y, Tortorici MA, Wall A, McGuire AT, Veesler D (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181:281–292
Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R (2020) COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res 24:91–98
Hamre D, Procknow JJ (1966) A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med 121:190–193
McIntosh K, Dees JH, Becker WB, Kapikian AZ, Chanock RM (1967) Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci USA 57:933–940
Tyrrell DA, Cohen S, Schlarb JE (1993) Signs and symptoms in common colds. Epidemiol Infect 111:143–156
Abdul-Rasool S, Fielding BC (2010) Understanding human coronavirus HCoV-NL63. Open Virol J 4:76–84
Fouchier RA, Hartwig NG, Bestebroer TM, Niemeyer B, de Jong JC, Simon JH, Osterhaus ADME (2004) A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci USA 101:6212–6216
Lau SK, Woo PC, Yip CC, Tse H, Tsoi HW, Cheng VC, Lee P, Tang BSF, Cheung CHY, Lee RA, So L, Lau Y, Chan K, Yuen K (2006) Coronavirus HKU1 and other coronavirus Infections in Hong Kong. J Clin Microbiol 44:2063–2071
Cheng VC, Lau SK, Woo PC, Yuen KY (2007) Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev 20:660–694
Devi LR, Raza MK, Musib D, Ramu V, Devi J, Roy M (2019) Nucleus targeting anthraquinone-based copper (II) complexes as the potent PDT agents: synthesis, photo-physical and theoretical evaluation. Inorg Chim Acta 500:119208
Musib D, Raza MK, Martina K, Roy M (2019) Mn(I)-based photoCORMs for trackable, visible light-induced CO release and photocytotoxicity to cancer cells. Polyhedron 172:125–131
Hilgenfeld R, Peiris M (2013) From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antiviral Res 100:286–295
Gao H, Yao H, Yang S, Li L (2016) From SARS to MERS: evidence and speculation. Front Med 10:377–382
Coleman CM, Frieman MB (2013) Emergence of the Middle East respiratory syndrome coronavirus. PLoSPathog 9:e1003595
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet 395:497–506
Risku M, Lappalainen S, Rasanen S, Vesikari T (2010) Detection of human coronaviruses in children with acute gastroenteritis. J Clin Virol 48:27–30
Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen K (2020) Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 9:221–236
Letko M, Marzi A, Munster V (2020) Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 5:562–569
Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, Lu L, Jiang S, Yang Z, Wu Y, Ying T (2020) Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect 9:382–385
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen H, Chen J, Luo Y, Guo H, Jiang R, Liu M, Chen Y, Shen X, Wang X, Zheng X, Zhao K, Chen Q, Deng F, Liu L, Yan B, Zhan F, Wang Y, Xiao G, Shi Z (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273
(a) http://www.nhc.gov.cn/jkj/s7915/202001/e4e2d5e6f01147e0a8df3f6701d49f33.shtml. (b) http://www.bigd.big.ac.cn/gwh/
Khailany RA, Safdar M, Ozaslan M (2020) Genomic characterization of a novel SARS-CoV-2. Gene Rep 19:100682
Yuen K, Ye Z, Fung S, Chan C, Jin D (2020) SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci 10:40
Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ (2020) A new coronavirus associated with human respiratory disease in China. Nature 579:265–269
Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA et al (2020) The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5:536–544
Paraskevis D, Kostaki EG, Magiorkinis G, Panayiotakopoulos G, Sourvinos G, Tsiodras S (2020) Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect, Genet Evol 79:104212
National Microbiology Data Center. (http://nmdc.cn/coronavirus)
Leng Z, Zhu R, Hou W, Feng Y, Liu H, Jin R, Jin K, Zhao RC (2020) Transplantation of ACE2—mesenchymal stem cells improves the outcome of patients with COVID-19 Pneumonia. Aging Dis 11:216–228
Toljan K (2020) Letter to the editor regarding the viewpoint “evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanism. ACS Chem Neurosci 11:1192–1194
Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q (2020) Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367:1444–1448
Siu YL, Teoh KT, Lo J, Chan CM, Kien F, Escriou N, Tsao SW, Nicholls JM, Altmeyer R, Peiris JSM, Bruzzone R, Nal B (2008) The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J Virol 82:11318–11330
Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F (2020) Structural basis of receptor recognition by SARS-CoV-2. Nature 581:221–224
Wan Y, Shang J, Graham R, Baric RS, Li F (2020) Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol 94:e00127-e220
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367:1260–1263
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D (2020) Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 20:S0092
Tan W, Zhao X, Ma X, Wang W, Niu P, Xu W, Gao GF, Wu G (2020) A novel coronavirus genome identified in a cluster of pneumonia cases—Wuhan, China 2019–2020. China CDC Weekly 2:61–62
Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, Lu L, Jiang S, Yang Z, Wu Y, Ying T (2020) Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect 9:382–385
Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF (2020) The proximal origin of SARS-CoV-2. Nat Med 26:450–452
Kikkert M (2020) Innate immune evasion by human respiratory RNA viruses. J Innate Immun 12:4–20
Kumar S, Maurya VK, Prasad AK, Bhatt MLB, Saxena SK (2020) Structural, glycosylation and antigenic variation between 2019 novel coronavirus (2019-nCoV) and SARS coronavirus (SARS-CoV). Virus Dis 31:13–21
Zhang Y, Geng X, Tan Y, Li Q, Xu C, Xu J, Hao L, Zeng Z, Luo X, Liu F, Wang H (2020) New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed Pharmacother 127:110195
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395:1033–1034
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler (2020) Structure, function, and antigenicity of the SARSCoV-2 Spike Glycoprotein. Cell 180:1–12
Wang Y, Wang Y, Chen Y, Qin Q (2020) Coronavirus infections and immune responses. J Med Virol 2020:1–9
Wang X, Xu W, Hu G, Xia S, Sun Z, Liu Z, Xie Y, Zhang R, Jiang S, Lu L (2020) SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol, 1–3
Tang B, Bragazzi NL, Li Q, Tang S, Xiao Y, Wu J (2020) An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov). Infect. Dis. Model 5:248–255
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367:1260–1263
Guo D (2020) Old weapon for new enemy: drug repurposing for treatment of newly emerging viral diseases. Virol Sin 35:253–255
Maxmen A (2020) More than 80 clinical trials launch to test coronavirus treatments. Nature 578:347–348
Ali A, Pooya A, Simani L (2020) Central nervous system manifestations of COVID-19: A systematic review. J Neurol Sci 413:116832
Ge XY (2013) Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503:535–538
Graham RL, Sparks JS, Eckerle LD, Sims AC, Denison MR (2008) SARS coronavirus replicase proteins in pathogenesis. Virus Res 133:88–10
Hurst KR, Koetzner CA, Masters PS (2013) Characterization of a critical interaction between the coronavirus nucleocapsid protein and nonstructural protein 3 of the viral replicase-transcriptase complex. J Virol 87:9159–9172
Koyama T, Platt D, Parida L (2020) Variant analysis of COVID-19 genomes, Bull. World Health Organ. Variant analysis of COVID-19 genomes. https://doi.org/10.2471/BLT.20.253591
Drosten C, Günther S, Preiser W, Werf S van der, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguière A, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra J, Müller S, Rickerts V, Stürmer M, Vieth S, Klenk H, Osterhaus ADME, Schmitz H, Doerr HW (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348:1967–1976
Cui J, Li F, Shi ZL (2019) Origin and evolution of pathogenic coronaviruses Nat. Rev Microbiol 17:181–192
(a) Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl Jr. J (2005) Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun 326:905–908. (b) Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271
Adhikari S, Meng S, Wu Y (2020) Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty 9:29
(a) Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R (2020) Coronavirus susceptibility to the antiviral remdesivir (GS-5734) Is mediated by the viral polymerase and the proofreading exoribonuclease MBIO 9:00221. (b) Yamamoto N, Matsuyama S., Hoshino T, Yamamoto N (2020) Nelfinavir inhibits replication of severe acute respiratory syndrome coronavirus 2 in vitro bioRxiv. https://doi.org/10.1101/2020.04.06.026476
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271
(a) Yamamoto N, Yang R, Yoshinaka Y, Amari S, Nakano T, Cinatl J, Rabenau H, Doerr HW, Hunsmann G, Otaka A, et al (2004) HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus Biochem Biophys Res Commun 318:719–725. (b) Wang X, Cao R, Zhang H, Liu J, Xu M, Hu H, Li Y, Zhao L, Li W, Sun X, Yang X, Shi Z, Deng F, Hu Z, Zhong W, Wang M (2020) The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discov 6:28
Uhlemann AC, Krishna S (2005) Antimalarial multi-drug resistance in Asia. Curr Top Microbiol Immunol 295:39–53
(a) Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S (2020) A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care 57:279–283. (b) Konig MF, Kim AH, Scheetz MH, Graef ER, Liew JW, Simard J, Machado PM, Gianfrancesco M, Yazdany J, Langguth D, Robinson PC (2020) Baseline use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19. Ann Rheum Dis 79:1386–1388
Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D (2020) In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 71:732–739. https://doi.org/10.1093/cid/ciaa237
Magagnoli J, Narendran S, Pereira F (2020) Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. (2020), DOI: https://doi.org/10.1101/2020.04.16.20065920.
Dong L, Hu S, Gao J (2020) Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther 14:58–60
Stockman LJ, Bellamy R, Garner P (2006) SARS: systematic review of treatment effects. PLos Med 3:e343
Fang L, Karakiulakis G, Roth M (2020) Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 8:e21
Gurwitz D (2020) Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res 81:537–540
Shah B, Modi P, Sagar SR (2020) In silico studies on therapeutic agents for COVID-19: Drug repurposing approach. Life Sci 252:117652
Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Stebbing J (2020) Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 395:e30–e31
Kim UJ, Won EJ, Kee SJ, Jung SI, Jang HC (2016) Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for Middle East respiratory syndrome. Antivir Ther 21:455–459
Kelleni MT (2020) Nitazoxanide/azithromycin combination for COVID-19: a suggested new protocol for early management. Pharmacol Res 157:104874
Haffizulla J, Hartman A, Hoppers M (2014) Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis 14:609–618
Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, de Barros e Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O (2020) Hydroxychloroquine with or without Azithromycin in mild-to-moderate Covid-19. N Engl J Med NEJMoa2019014
Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honore S, Colson P, Chabriere E, Scola BL, Rolain JM, Brouqui P, Raoult D (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 56:105949
Hung IF, Lung K, Tso EY, Liu R, Chung TW, Chu M, Ng Y, Lo J, Chan J, Tam AR, Shum H, Chan V, Wu AK, Sin K, Leung W, Law W, Lung DC, Sin S, Yeung P, Yip CC, Zhang RR, Fung AY, Yan EY, Leung K, Daniel J, Chu AW, Chan W, Ng AC, Lee R, Fung K, Yeung A, Wu T, Chan JW, Yan W, Chan W, Chan JF, Lie AK, Tsang OT, Cheng VC, Que T, Lau C, Chan K, To KK, Yuen K (2020) Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. The Lancet 395:1695–1704. https://doi.org/10.1016/S0140-6736(20)31042-4
(a) Banerjee S, Chakravarty AR (2015) Metal complexes of curcumin for cellular imaging, targeting, and photoinduced anticancer activity. Acc Chem Res 48:2075–2083. (b) Sadler PJ, Li H, Sun H (1999) Coordination chemistry of metals in medicine: target sites for bismuth. Coord Chem Rev 185:689–709
Imberti C, Zhang P, Huang H, Sadler PJ (2020) New designs for phototherapeutic transition metal complexes. Angew Chem Int Ed 59:61–73
García-Gallego S, Serramía MJ, Arnaiz E, Díaz L, Muñoz-Fernández MA, Gómez-Sal P, Ottaviani MF, Gómez R, de la Mata FJ (2011) Transition-metal complexes based on a sulfonate-containing N-donor ligand and their use as HIV antiviral agents. Eur J Inorg Chem 2011:1657–1665
Thompson KH, Orvig C (2001) Coordination chemistry of vanadium in metallopharmaceutical candidate compounds. Coord Chem Rev 219:1033–1053
D’Cruz OJ, Dong Y, Uckun FM (2003) Potent dual anti-HIV and spermicidal activities of novel oxovanadium(V) complexes with thiourea non-nucleoside inhibitors of HIV-1 reverse transcriptase. Biochem Biophys Res Commun 302:253–264
Sun RW, Ma D, Wong EL, Che C (2007) Some uses of transition metal complexes as anti-cancer and anti-HIV agents. Dalton Trans 43:4884–4892
d Aguiar I, d Santos ER, Mafud AC, Annies V, Navarro-Silva MA, Malta VRdS, Gambardella MTdP, Marques FdA, Carlos RM (2017) Synthesis and characterization of Mn(I) complexes and their larvicidal activity against Aedes aegypti, vector of dengue fever. Inorg Chem Commun 84:49−55
Song R, Witvrouw M, Schols D, Robert A, Bolzorini J, Clercq E De, Bernodou J, Meunier B (1997) Anti-HIV activities of anionic metalloporphyrins and related compounds. Antivir Chem Chemother 8:85-97
Vausselin T, Calland N, Belouzard S, Descamps V, Douam F, Helle F, François C, Lavillette D, Duverlie G, Wahid A, Fénéant L, Cocquerel L, Guérardel Y, Wychowski C, Biot C, Dubuisson J (2013) The antimalarial ferroquine is an inhibitor of hepatitis C virus. Hepatology 58:86–97
Biot C, Daher W, Chavain N, Fandeur T, Khalife J, Dive D, Clercq ED (2006) Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem 49:2845–2849
Wang H, Li Z, Niu J, Xu Y, Ma L, Lu A, Wang X, Qian Z, Huang Z, Jin X, Leng Q, Wang J, Zhong J, Sun B, Meng G (2018) Antiviral effects of ferric ammonium citrate. Cell Discov 4:14
Gadhachanda VR, Eastman KJ, Wang Q, Phadke AS, Patel D, Yang W, Marlor CW, Deshpande M, Huang M, Wiles JA (2018) Ferrocene-based inhibitors of hepatitis C virus replication that target NS5A with low picomolar in vitro antiviral activity. Bioorg Med Chem Lett 28:3463–3471
Belema M, Nguyen VN, Bachand C, Deon DH, Goodrich JT, James CA, Lavoie R, Lopez OD, Martel A, Romine JL, Ruediger EH, Snyder LB, St. Laurent DR, Yang F, Zhu J, Wong HS, Langley DR, Adams SP, Cantor GH, Chimalakonda A, Fura A, Johnson BM, Knipe JO, Parker DD, Santone KS, Fridell RA, Lemm JA, O’Boyle DR, Colonno RJ, Gao M, Meanwell NA, Hamann LG. J Med Chem 28:2013–2032
Asbell PA, Epstein SP, Wallace JA, Epstein D, Stewart CC, Burger MR (1998) Efficacy of cobalt chelates in the rabbit eye model for epithelial herpetic keratitis. Cornea 17:550–557
Epstein SP, Pashinsky YY, Gershon D, Winicov I, Srivilasa C, Kristic KJ, Asbell PA (2006) Efficacy of topical cobalt chelate CTC-96 against adenovirus in a cell culture model and against adenovirus keratoconjunctivitis in a rabbit model. BMC Ophthalmol 6:22
Chang EL, Simmers C, Knight DA (2010) Cobalt Complexes as antiviral and antibacterial agents. Pharmaceuticals (Basel) 3:1711–1728
Saini AK, Kumari P, Sharma V, Mathur P, Mobin SM (2016) Varying structural motifs in the salen based metal complexes of Co(ii), Ni(ii) and Cu(ii): synthesis, crystal structures, molecular dynamics and biological activities Dalton Trans 45:19096−19108
Delehanty JB, Bongard JE, Thach DC, Knight DA, Hickey TE, Chang EL (2008) Antiviral properties of cobalt(III)-complexes. Bioorg Med Chem 16:830–837
Cígler P, Kožíšek M, Řezáčová P, Brynda J, Otwinowski Z, Pokorná J, Plešek J, Grüner B, Dolečková-Marešová L, Máša M, Sedláček J, Bodem J, Kräusslich HG, Král V, Konvalinka J (2005) From nonpeptide toward noncarbon protease inhibitors: Metallacarboranes as specific and potent inhibitors of HIV protease. Proc Natl Acad Sci USA 102:15394–15399
Řezáčová P, Pokorná J, Brynda J, Kožíšek M, Cígler P, Lepšík M, Fanfrlík J, Řezáč J, Šašková KG, Sieglová I, Plešek J, Šícha V, Grüner B, Oberwinkler H, Sedláček J, Kräusslich HG, Hobza P, Král V, Konvalinka J (2009) Design of HIV Protease Inhibitors Based on Inorganic Polyhedral Metallacarboranes. J Med Chem 52:7132–7141
García-Gallego S, Jesús Serramía M, Arnaiz E, Díaz L, Muñoz-Fernández MA, Gómez-Sal P, Ottaviani MF, Gómez R, Mata FJDL (2011) Transition-metal complexes based on a sulfonate-containing N-donor ligand and their use as HIV antiviral agents. Eur J Inorg Chem, 1657–1665
Rogolino D, Carcelli M, Bacchi A, Compari C, Contardi L, Fisicaro E, Gatti A, Sechi M, Stevaert A, Naesens L (2015) A versatile salicyl hydrazonic ligand and its metal complexes as antiviral agents. J Inorg Biochem 150:9–17
Loginova NV, Koval’chuk TV, Polozov GI, Osipovich NP, Rytik PG, Kucherov II, Chernyavskaya AA, Sorokin VL, Shadyro OI (2008) Synthesis, characterization, antifungal and anti-HIV activities of metal(II) complexes of 4,6-di-tert-butyl-3-[(2-hydroxyethyl)thio]benzene-1,2-diol. Eur J Med Chem 43:1536–1542
Hunter TM, McNae IW, Simpson DP, Smith AM, Moggach S, White F, Walkinshaw MD, Parsons S, Sadler PJ (2007) Configurations of Nickel-Cyclam antiviral complexes and protein recognition. Chem Eur J 13:40–50
Bitu MNA, Hossain MS, Zahid AASM, Zakaria CM, Kudrat-E-Zahan M (2019) Anti-pathogenic activity of Cu(II) complexes incorporating Schiff bases: a short review. Am J Heterocycl Chem 5:11–23
Zerda KS, Gerba CP, Goyel SM (1985) Adsorption of viruses to charge-modified silica. Appl Environ Microbial 49:91–95
Ishida T (2016) Bacteriolyses of Cu2+ solution on bacterial cell walls/cell membrane and DNA base-pairing damages. Biomed Res Trace Elemt 27:151–161
Srivastava A (2009) Antiviral activity of copper complexes of isoniazid against RNA tumor viruses. Resonance 14:754–760
Pelosi G, Bisceglie F, Bignami F, Ronzi P, Schiavone P, Carla RM, Casoli C, Pilotti E (2010) Antiretroviral activity of thiosemicarbazone metal complexes. J Med Chem 53:8765–8769
Chauhan G, Rath G, Goyal AK (2013) Non-invasive systemic drug delivery through mucosal routes. Nanomedicine, Biotechnol Int J 41:4
García-Gallego S, Sánchez Rodríguez J, Luis Jiménez J, Cangiotti M, Francesca Ottaviani M, Muñoz-Fernández MÁ, Gómez R, Mata FJDL (2012) Polyanionic N-donor ligands as chelating agents in transition metal complexes: synthesis, structural characterization and antiviral properties against HIV. Dalton Trans 41:6488–6499
Dorotíková S, Kožíšková J, Malček M, Jomová K, Herich P, Plevová K, Briestenská K, Chalupková A, Mistríková J, Milata V, Dvoranová D, Bučinský L (2015) Tetracarboxylatoplatinum(IV) complexes featuring monodentate leaving groups—a rational approach toward exploiting the platinum(IV) prodrug strategy. J Inorg Biochem 150:160–173
McGuire KL, Hogge J, Hintze A, Liddle N, Nelson N, Pollock J, Brown A, Facer S, Walker S, Lynch J, Harrison RG, Busath DD (2019) Copper complexes as influenza antivirals: reduced zebrafish toxicity. Eng Nanomater - Health Saf. https://doi.org/10.5772/intechopen.88786
Kar M, Khan NA, Panwar A, Bais SS, Basak S, Goel R, Sopory S, Medigeshi GR (2019) Zinc chelation specifically inhibits early stages of dengue virus replication by activation of NF-κB and induction of antiviral response in epithelial cells. Zinc chelation specifically inhibits early stages of dengue virus replication by activation of NF-κB and induction of antiviral response in epithelial cells. Front Immunol 10:2347
Krenn BM, Gaudernak E, Holzer B, Lanke K, Van Kuppeveld FJM, Seipelt J (2009) Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J Virol 83:58–64
Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ (2010) Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog 6:1001176
Arens M, Travis S (2000) Detection of Legionella pneumophila using a real-time PCR hybridization assay. J Clin Microbiol 38:1758–1762
Hsu JTA, Kuo C, Hsieh H, Wang Y, Huang K, Lin CPC, Huang P, Chen X, Liang P (2004) Evaluation of metal-conjugated compounds as inhibitors of 3CL protease of SARS-CoV. FEBS Lett 574:116–120
Karaküçük-Iyidogan A, Tasdemir D, Oruç-Emre EE, Jan B (2011) Novel platinum(II) and palladium(II) complexes of thiosemicarbazones derived from 5-substitutedthiophene-2-carboxaldehydes and their antiviral and cytotoxic activities. Eur J Med Chem 46:5616–5624
Simic V, Kolarevic S, Brceki I, Jeremic D, Vukovic-gacic B (2016) Cytotoxicity and antiviral activity of palladium(II) and platinum(II) complexes with 2-(diphenylphosphino)benzaldehyde 1-adamantoylhydrazone. Turk J Biol 40:661–669
Genova P, Varadinova T, Matesanz AI, Marinova D, Souza P (2004) Toxic effects of bis(thiosemicarbazone) compounds and its palladium(II) complexes on herpes simplex virus growth. Toxicol Appl Pharmacol 197:107–112
Kovala-Demertzi D, Varadinova T, Genova P, Souza P, Demertzis MA (2007) Platinum(II) and palladium(II) complexes of pyridine-2-carbaldehyde thiosemicarbazone as alternative antiherpes simplex virus agents. Bioinorg Chem Appl 2007:56165
Cavicchioli M, Massabni AC, Heinrich TA, Costa-Neto CM, Abrão EP, Fonseca AL, Castellano EE, Corbi PP, Lustri WR, Leite CQF (2010) Pt(II) and Ag(I) complexes with acesulfame: crystal structure and a study of their antitumoral, antimicrobial and antiviral activities. J Inorg Biochem 104:533–540
Allardyce CS, Dyson PJ, Ellis DJ, Salter PA, Scopelliti R (2003) Synthesis and characterisation of some water soluble ruthenium(II)–arene complexes and an investigation of their antibiotic and antiviral properties. J Organomet Chem 668:35–42
Luedtke NW, Hwang JS, Glazer EC, Gut D, Kol M, Tor Y (2002) Eilatin Ru(II) complexes display anti-HIV activity and enantiomeric diversity in the binding of RNA. Chem Bio Chem 3:766–771
Mishra L, Singh AK, Trigun SK, Singh SK, Pandey SM (2004) J Exp Biol 42:660–666
Anchuri SS, Gangarapu K, Thota S, Karki SS, Clercq ED, Andrei G, Snoeck R, Balzarini J (2016) Evaluation of hepatoprotective and antioxidant activity of newly synthesized Ho(III) complex. Biointerface Res Appl Chem 6:1491–1496
Gunatilleke SS, Barrios AM (2006) Inhibition of lysosomal cysteine proteases by a series of Au(I) complexes: a detailed mechanistic investigation. J Med Chem 49:3933–3937
Gunatilleke SS, de Oliveira CAF, McCammon JA, Barrios AM (2008) The ruthenium(II)-arene compound RAPTA-C induces apoptosis in EAC cells through mitochondrial and p53-JNK pathways. J Biol Inorg Chem 13:555–561
Okada T, Patterson BK, Ye SQ, Gurney ME (1993) Silencing the alarms: Innate immune antagonism by rotavirus NSP1 and VP3. Virology 192:631–642
Sun RWY, Yu WY, Sun H, Che CM (2004) In vitro inhibition of human immunodeficiency virus type-1 (HIV-1) reverse transcriptase by gold(III) porphyrins. Chem Bio Chem 5:1293–1298
Fonteh P, Meyer D (2009) Novel gold(i) phosphine compounds inhibit HIV-1 enzymes. Metallomics 1:427–433
Fonteh PN, Keter FK, Meyer D (2010) Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Bio. Metals 23:185–196
Qasim M, Baipaywad P, Udomluck N, Na D, Park H (2014) Enhanced therapeutic efficacy of lipophilic amphotericin B against Candida albicans with amphiphilic poly(N-isopropylacrylamide) nanogels. Macromol Res 22:1125–1131
Gurunathan S, Qasim M, Park C, Yoo H, Kim JH, Hong K (2018) Cytotoxic potential and molecular pathway analysis of silver nanoparticles in human colon cancer cells HCT116. Int J Mol Sci 19:2269
Jeyaraj M, Gurunathan S, Qasim M, Kang MH, Kim JH (2019) A comprehensive review on the synthesis, characterization, and biomedical application of platinum nanoparticles. Nanomaterials 9:1719
Chakravarthy KV, Bonoiu AC, Davis WG, Ranjan P, Ding H, Hu R, Bowzard JB, Bergey EJ, Katz JM, Knight PR (2010) Gold nanorod delivery of an ssRNA immune activator inhibits pandemic H1N1 influenza viral replication. Proc Natl Acad Sci USA 107:10172–10177
Lee MY, Yang JA, Jung HS, Beack S, Choi JE, Hur W, Koo H, Kim K, Yoon SK, Hahn SK (2012) Hyaluronic acid-gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection. ACS Nano 6:9522–9531
Halder A, Das S, Ojha D, Chattopadhyay D, Mukherjee A (2018) Highly monodispersed gold nanoparticles synthesis and inhibition of herpes simplex virus infections. Mater Sci Eng C 89:413–421
Papp I, Sieben C, Ludwig K, Roskamp M, Böttcher C, Schlecht S, Herrmann A, Haag R (2010) Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small 6:2900–2906
Hu R, Li S, Kong F, Hou R, Guan X, Guo F (2014) Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genet Mol Res 13:7022–7028
Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C (2010) Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol 8:1–10
Mohammed Fayaz A, Ao Z, Girilal M, Chen L, Xiao X, Kalaichelvan PT, Yao X (2012) Inactivation of microbial infectiousness by silver nanoparticles-coated condom: a new approach to inhibit HIV- and HSV-transmitted infection. Int J Nanomed 7:5007–5018
Mori Y, Ono T, Miyahira Y, Nguyen VQ, Matsui T, Ishihara M (2013) Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza a virus. Nanoscale Res Lett 8:93
Ghosal K, Sarkar K (2018) Biomedical applications of graphene nanomaterials and beyond. ACS Biomater Sci Eng 4:2653–2703
Song Z, Wang X, Zhu G, Nian Q, Zhou H, Yang D, Qin C, Tang R (2015) Virus capture and destruction by label-free graphene oxide for detection and disinfection applications. Small 11:1171–1176
Sametband M, Kalt I, Gedanken A, Sarid R (2014) Herpes simplex virus type-1 attachment inhibition by functionalized graphene oxide. ACS Appl Mater Interfaces 6:1228–1235
Yang XX, Li CM, Li YF, Wang J, Huang CZ (2017) Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale 9:16086–16092
Du T, Cai K, Han H, Fang L, Liang J, Xiao S (2015) Probing the interactions of CdTe quantum dots with pseudorabies virus. Sci Rep 5:1–10
Huang S, Gu J, Ye J, Fang B, Wan S, Wang C, Ashraf U, Li Q, Wang X, Shao L (2019) Benzoxazine monomer derived carbon dots as a broad-spectrum agent to block viral infectivity. J Colloid Interface Sci 542:198–206
Antoine TE, Mishra YK, Trigilio J, Tiwari V, Adelung R, Shukla D (2012) Prophylactic, therapeutic and neutralizing effects of zinc oxide tetrapod structures against herpes simplex virus type-2 infection. Antivir Res 96:363–375
Antoine TE, Hadigal SR, Yakoub AM, Mishra YK, Bhattacharya P, Haddad C, Valyi-Nagy T, Adelung R, Prabhakar BS, Shukla D (2016) Intravaginal zinc oxide tetrapod nanoparticles as novel immunoprotective agents against genital herpes. J Immunol 196:4566–4575
Tejman-Yarden N, Miyamoto Y, Leitsch D, Santini J, Debnath A (2013) A reprofiled drug, auranofin, is effective against metronidazole-resistant Giardia lamblia. Chemother 57:2029–2035
Harbut MB, Vilcheze C, Luo X, Hensler ME, Guo H, Yang B, Chatterjee AK, Nizet V, Jacobs JWR, Schultz PG, Wang F (2015) Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc Natl Acad Sci USA 112:4453–4458
Musib D, Raza MK, Pal M, Roy M (2021) A red light-activable MnI(CO)3-functionalized gold nanocomposite as the anticancer prodrug with theranostic potential. Appl Organomet 35:e6110
Musib D, Banerjee S, Garai A, Soraisam U, Roy M (2018) Synthesis, theory and in vitro photodynamic activities of new copper(II)-histidinito complexes. Chemistry Select 3:2767–2775
Musib D, Pal M, Raza MK, Roy M (2020) Photo-physical, theoretical and photo-cytotoxic evaluation of a new class of lanthanide(iii)–curcumin/diketone complexes for PDT application. Dalton Trans 49:10786–10798
Marzo T, Messori L, Marzo T, Messori L (2020) A role for metal-based drugs in fighting COVID-19 infection? The case of auranofin. ACS Med Chem Lett 11:1067–1068
Rothan HR, Stone S, Natekar J, Kumari P, Arora K, Kumar M (2020) The FDA-approved gold drug Auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. bioRxiv preprint. https://doi.org/10.1101/2020.04.14.041228
Gil-Moles M, Basu U, Büssing R, Hoffmeister H, Türck S, Varchmin A, Ott I (2020) Gold metallodrugs to target coronavirus proteins: inhibitory effects on the spike-ACE2 interaction and on PLpro protease activity by auranofin and gold organometallics. Chem Eur J 26:15140–15144
Milenković DA, Dimić DS, Avdovićac EH, Marković ZS (2020) Several coumarin derivatives and their Pd(II) complexes as potential inhibitors of the main protease of SARS-CoV-2, an in silico approach. RSC Adv 10:5099–35108
Haribabu J, Srividya S, Mahendiran D, Gayathri D, Venkatramu V, Bhuvanesh N, Karvembu R (2020) Synthesis of palladium(II) complexes via Michael addition: antiproliferative effects through ROS-mediated mitochondrial apoptosis and docking with SARS-CoV-2. Inorg Chem 59:17109–17122
Pal M, Musib D, Roy M (2021) Transition metal complexes as potential tools against SARS-CoV-2: an in silico approach. New J Chem 45:1924–1933
Yadav M, Dhagat S, Eswari JS (2020) Emerging strategies on in silico drug development against COVID-19: challenges and opportunities. Eur J Pharm Sci 155:105522
El Hawary SS, Khattab AR, Marzouk HS, El Senousy AS, Alex MGA, Aly OM, Telebe M, Abdelmohsen UR (2020) In silico identification of SARS-CoV-2 spike (S) protein–ACE2 complex inhibitors from eight Tecoma species and cultivars analyzed by LC-MS. RSC Adv 10:43103–43108
Maffucci I, Contini A (2020) In silico drug repurposing for SARS-CoV-2 main proteinase and spike proteins. J Proteome Res 19:4637–4648
Sportelli MC, Izzi M, Kukushkina EA, Hossain SI, Picca RA, Ditaranto N, Cioffi N (2020) can nanotechnology and materials science help the fight against SARS-CoV-2? Nanomaterials (Basel) 4:802
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Musib, D., Pal, M., Allam, U.S., Roy, M. (2022). Brief History, Pathophysiology, Transmission of SARS-CoV-2 Virus, and Recent Advances on Transition Metal Complexes and Nanocomposites as the Potent Antiviral Agents from COVID-19 Perspectives. In: Swain, B.P. (eds) Nanostructured Biomaterials. Materials Horizons: From Nature to Nanomaterials. Springer, Singapore. https://doi.org/10.1007/978-981-16-8399-2_1
Download citation
DOI: https://doi.org/10.1007/978-981-16-8399-2_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8398-5
Online ISBN: 978-981-16-8399-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)