Abstract
The chemical industry contributes to 6% of global anthropogenic greenhouse gas (GHG) emissions. A handful of chemical processes (ammonia, nitric acid, methanol, olefins, aromatics, and chlor-alkali) account for 65% of those emissions. Decarbonization of the chemical industry will depend on addressing the intermittency of renewable electricity possibly via low-carbon hydrogen production using water electrolysis. A low-carbon power grid, which could happen in the next decade, would enable the chemical industry to reduce its GHG emissions by at least 35 percent. The remaining heat-based and direct emissions could be addressed by direct use of low-carbon electricity for heat or by generating hydrogen that can be used as a fuel and reducing agent coupled with CO2 capture and utilization efforts. Herein, we discuss how materials innovations could enable the transition to a lower carbon future when based on first-principles and economic realities.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
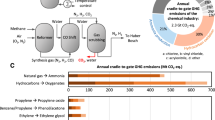
Anthropogenic greenhouse gas (GHG) emissions are generated mostly from burning fossil carbon (coal, natural gas and oil) for energy, transportation, agriculture, and industrial activity.1 Nearly one third of all anthropogenic GHG emissions (16.1 billion tons of CO2eq) were from industrial activity in 2010 (Figure 1a).2 The chemical industry, including fertilizer production, accounts for 19% of all industrial emissions (3.1 billion tons CO2eq).2 Global chemical industrial emissions can be attributed to four separate value chains: feedstocks and fuels (hydrocarbons and coal-production-related emissions from the oil and gas industry); raw materials (ammonia, nitric acid, methanol, olefins, aromatics, chlor-alkali, and sulfuric acid); intermediates (plastic resins and other chemicals); and final products (provided by brand owners).
Global GHG emissions breakdown2 (a) and our estimate of chemical industry emissions separated into four value chains (b) and amount of materials consumed (c). See Tables S3, S6, S9–10 and associated sections in the SI for details.
We estimated that all of the global chemical industry, including fertilizer production, emitted 2.6 billion tons CO2eq in 2019 according to cradle-to-gate LCA methodology using the ecoinvent database3 (Figure 1b, see Table S10 in Supplementary information [SI] for details). Emissions from electricity use accounted for 35% of all emissions across the value chain, increasing as materials flowed from feedstocks and fuels (19%), raw materials (18%), and intermediates (59%) to final products (77%). The US Department of Energy manufacturing survey estimated similar GHG emissions from electricity (43%) used by the US chemical industry.4 We estimated that process heat emissions resulting from burning fossil fuels account for 23% of the emissions and the majority of that happens during raw material production of ammonia, methanol, and olefins, which are all high-temperature processes. Direct emissions account for 42% of all emissions. The majority of the direct emissions happen either during raw material production or extraction and refining of feedstocks and fuels. Direct emissions from raw materials are mainly from the ammonia process during hydrogen generation from methane or coal and nitrogen oxide emissions from the nitric acid process. Feedstock direct GHG emissions are mainly from leaked and flared methane gas from extraction processes, which could be reduced with global regulations and commitments from oil and gas companies.
The chemical industry is estimated to use a total 1.2 billion tons of various hydrocarbons from natural gas, petroleum and coal as feedstocks (70% of the total), and fuels (30% of the total) to produce a total 1.2 billion tons of raw materials (Figure 1c). The fossil carbon used as feedstocks and fuels by the chemical industry accounts for 5% of global fossil carbon consumption. Other than hydrocarbon feedstocks, nitrogen separated from air is used in ammonia synthesis, sodium chloride is mined and used in the chlor-alkali process, providing chlorine gas and sodium hydroxide (caustic), and sulfur, byproduct from removal of contaminants in petroleum and natural gas, is burned to make sulfuric acid. Nearly all of the olefins, aromatics, and methanol along with some of the chlor-alkali products, ammonia, nitric and sulfuric acid are converted to 0.5 billion tons of intermediates (mainly plastics) by the chemical industry. The remaining ammonia and sulfuric and nitric acids are converted to fertilizers used by the agriculture industry. Various other industrial processes, such as metal, paper, and textile production and water treatment, also use some of the chlor-alkali products and sulfuric and nitric acids.
There are three main energy intensive chemical processes (reforming, gasification, and steam cracking) that are hard to abate because they have large direct emissions and/or need high-temperature heat. These processes provide a handful of raw materials (ammonia, methanol, olefins, and aromatics) that are the building blocks for all intermediates in the chemical industry. Herein, we focus on materials innovations to enable further use of low-carbon electricity in these difficult to abate chemical processes. We note that improving efficiency of these processes via materials innovation is also crucial to the decarbonization effort along with electrification of the chemical industry. We identified four key areas (boxes in red in Figure 2) that could benefit from materials innovation: direct electricity use in high-temperature endothermic processes, low-carbon hydrogen generation, CO2 capture and utilization (CCU), and new electrochemical processes.
Direct use of renewable electricity
Over the last decade, low-carbon electricity (mainly solar and wind) has become cost-competitive to fossil-based electricity.5,6 As the lower carbon electricity becomes more reliable and widely deployed, the chemical industry would decarbonize by switching to it, eliminating nearly 35% of the emissions from electricity use (Figure 1b). More specifically, the chlor-alkali process, providing chlorine gas and caustic, and air separations, providing nitrogen and oxygen gas, rely mostly on electricity, emitting 174 and 183 million tons CO2eq, respectively, (together representing 13% of the total of chemical industry emission). The majority of oxygen gas is used in gasification of coal, which is practiced in Asia. If coal use could be reduced or eliminated, that would decrease the energy need for oxygen gas. There are opportunities for materials innovations especially in membranes7 and sorbent materials8,9 that could make the separations more efficient, reducing total energy not only for air separations but also other large industrial separations such as olefins/paraffins. Currently, nearly half of all energy in the US industry is used for separations.10
The remaining chemical process emissions are from use of heat and steam to drive endothermic reactions, separations, and direct emissions from the processes. Differences in enthalpies between inlet and exit streams lead to heat being absorbed or released as a chemical transformation is driven in the desired direction. In the steam cracking process, the enthalpy difference between a fossil fuel and its combustion products drives the enthalpy changes of the desired and undesired reactions.11 The operating temperature is chosen so the Gibbs energy change of the reaction is negative, making the reaction spontaneous.
The steam cracking process provides olefinic and aromatic raw materials and was estimated to emit 236 million tons of CO2eq globally in 2019 (9% of total from the chemical industry). Steam cracking is initiated by thermally breaking C–C and C–H bonds (above 800°C), forming radicals, which propagate, isomerize, and terminate to form a distribution of products (olefins and aromatics) and byproducts (hydrogen, methane, higher hydrocarbons) that depend on the type of feedstock and furnace run conditions, such as temperature and residence time (Figure 3a).11 Byproduct methane and hydrogen are separated and usually fed back into the furnace as fuel gas (Figure 3b). Lighter hydrocarbon byproducts and unconverted raw materials are recycled into the furnace as a feedstock, and pyrolysis oil is burned or sent back to refineries.
Sankey diagram of steam cracking of hydrocarbon amounts (numbers are in million tons per year) to olefin and aromatic raw materials (a), total annual energy (4.8 billion GJ) breakdown by source (b) and use12 (c), CO2 emissions by source (d); see the SI for details.
Theoretical energy requirements for ethylene from ethane cracking are estimated as 4.9 GJ/ton ethylene using the standard enthalpies of formation at 25°C (Equation 1):
Total energy for the production of olefins and aromatics is reported in the range of 14–19 GJ/ton of valuable products.12 The discrepancy between theory and actual is due to energy needed for separations and purification of final products, which utilizes the high pressure steam generated from the latent heat in the furnace. So, only a quarter (23%) of the total energy consumed goes to the heat of reaction, 31% goes to separations, 22% to compression, and the rest is lost (Figure 3c).12 Emission from the process comes from the use of byproduct methane and hydrogen as a fuel except in the case of ethane, which needs additional methane for steam generation (Figure 3d). Separations and compression could be decarbonized since they can potentially run on low-carbon electrically driven motors, but high-temperature heating above 800°C still has challenges and could be improved with innovative materials or processes.13,14
An alternative approach to olefins and aromatics production is to reduce the temperature requirement for the process using catalysis, which is now practiced at the commercial scale especially for propylene production using fluid catalytic cracking in refineries and propane dehydrogenation in petrochemical operations.15 Most of the aromatics, especially toluene and xylenes, are mainly made in refineries using catalytic reforming at lower temperatures.16 Materials research for catalytic processes that are more specific toward ethylene has the potential to eliminate byproducts such as methane and to reduce operating temperatures, enabling the use of renewable power as a heating source.17,18 To that end, commercial methanol-to-olefins (MTO) processes using zeolite catalysis can be performed at lower temperatures, providing an ethylene/propylene mixture at high yield or mostly propylene at moderate yields.19 However, the carbon intensity of the MTO process strongly depends on the feedstock type, where the currently practiced coal-based MTO process (12 CO2eq/olefin)20 generates nearly eight times more GHG than steam cracking (1.5 CO2eq/olefin).21
The MTO route would be more favorable with lower carbon methanol made from gasification of waste streams like plastic and biomass or high-concentration CO2 from industrial emissions, which all require additional low-carbon hydrogen. To that end, a methanol economy22 was proposed as a transition to a low-carbon chemical industry away from hydrocarbon feedstocks that require cost-competitive and readily available low-carbon hydrogen at a massive scale.
Since most of the electricity-based processes and low heat use are on the intermediates and products that can be decarbonized along with the grid, this leaves four main processes (ammonia, methanol, olefins, and aromatics) emitting about 1.3 billion tons CO2eq (50% of the total from chemical industry) from direct emissions and heat that can benefit from low-carbon hydrogen coupled with CO2 utilization and storage.
Low-carbon H2
Hydrogen, despite being the most widely available element in the universe and fuel for the stars, exists only as bonded with other elements on Earth, such as in water, hydrocarbons, and biomass. Hydrogen is commercially used at a large scale (115 million tons hydrogen total, 70 million tons pure and 45 million tons mixed with other gases in 2018) and produced from mostly reforming of hydrocarbons and gasification of coal, emitting about 830 million tons of direct CO2eq.23 Reforming is an endothermic reaction (Equation 2) and requires temperatures above 800°C, which are usually obtained by burning part of the feedstock, for example oxy-firing of methane and gasification of coal in ammonia production. Fossil carbon-based hydrogen production leads to 10 and 19 kg CO2eq/kg H2 from methane and coal, respectively. The cost of hydrogen production is in the range of $1–1.7/kg H2 depending on the cost of the fossil carbon source. Low-carbon hydrogen could be produced by CO2 capture and storage (CCS) at a cost $1.5–2.2/kg H2.23
An alternative option is to generate H2 from water electrochemically, which can be done using existing commercial electrolysis technologies. However, the thermodynamics of water splitting to generate H2 is not favorable when compared to the steam methane reforming route, requiring nearly 7 times more energy (Equation 3). Thus, H2 from water splitting using the current power grid is estimated to cost $5/kg H2 and emit 40 kg CO2eq/kg H2. (All of the emission burden is allocated to H2.)23,24 Moreover, the cost of commercial electrolyzers is still in the range of $1000/kW, which accounts for $1/kg H2 CAPEX cost. These are the main reasons that only a small fraction of global H2 production is currently from dedicated water electrolysis units.
Today, solar and wind account for a small fraction (7%) of global electricity even with record breaking deployment of 250 GW solar and wind new capacity added in 2020.6 If solar and wind can be deployed at four times the latest capacity additions25 then the scenario for low-carbon hydrogen may change in the next decade with solar photovoltaics and onshore wind providing the lowest cost electricity without incentives.5,6 Thus, some projected that water splitting to make low-carbon H2 (1.0 kg CO2eq/kg H2, all emission burden is allocated to H2)26 could become cost-competitive at $1.5/kg H2 when there is an abundant source of low-carbon electricity at $20/MWh cost and with the electrolyzer installed system cost below $300/kW (70% lower than current cost) running at least at 50% capacity with 10 years lifetime.24 At this point, low-carbon H2 would be not only energy carrier but also storage media and reducing agent for CO2 utilization efforts.27 However, CO2 utilization into raw materials using low-carbon hydrogen would make sense only after more efficient use of renewable electricity is satisfied.28,29 Co-product O2 from water electrolysis would reduce the emission burden on H2 when emissions are allocated to both products based on their mass or economic value. Because, O2 can be used in oxy-firing of methane, producing pure CO2 and thus dramatically reducing the cost of CO2 capture.
There are other ways to generate low-carbon hydrogen. Methane pyrolysis has favorable thermodynamics (Equation 4) but still is in the development stage to find an efficient catalytic route.30 Biomass and waste plastic gasification is another route to low-carbon hydrogen given the fact that the non-biogenic portion of waste CO2 would still need to be captured and utilized or stored.
Currently, nearly half of the pure H2 produced (31 million tons) is used for ammonia production, which is the main ingredient of synthetic fertilizers.23 Using low-carbon hydrogen enabled by renewable electricity in the ammonia process can reduce GHG emissions from ammonia production by more than 90 %.26 Another 12 million tons of H2 are generated as a mixture of syngas, which is directly converted to methanol.23 Methanol can also be synthesized directly from CO2 and hydrogen. This route has been proven to work at a small commercial scale, while multiple larger scale projects are planned.22 Total emissions savings of ammonia and methanol made from low carbon would be 570 million tons of CO2eq (21% of the total of chemical industry), requiring nearly 3 times more energy compared to conventional process.21
CO2 feedstock used in the methanol process would be initially captured from high-concentration emitting processes, such as hydrogen, cement and steel production.31 The cost of CO2 capture based on commercial amine-scrubbing technology is equal to the European Trading System (ETS) cost of CO2 of $55/ton. However, CO2 direct air capture (DAC) would be the final solution to close the loop on anthropogenic emissions. Materials innovations32 to capture CO2 directly from air efficiently could avoid the CO2 transport infrastructure and could enable deployment of CO2 utilization technologies at remote places where solar and wind power are readily available. Methanol from CO2 would also have a negative carbon footprint (− 0.67 kg CO2eq/kg methanol) according to cradle-to-gate LCA methodology. Converting this methanol to olefins and aromatics could generate carbon negative materials with − 1.8 kg CO2eq/kg olefins or aromatics, but this would require 96 GJ/ton olefin and 171 GJ/ton aromatics energy, which is much more than conventional processes (17 GJ/ton olefin and 7 GJ/ton aromatic).21 The cost of reducing 210 million tons CO2 emission by the CCU route was estimated to be €27 billion per year till 2050 for the European chemical industry.21 If we project this to 2.6 billion tons CO2eq (our estimate for global chemical industry, Table S10) reduction to zero via CCU, it would cost €334 billion per year till 2050.
Even when economics align with low-carbon hydrogen, there are still challenges around storage and transportation. Being the lightest and the smallest element, hydrogen could use up to 35% of its energy content to be compressed or liquefied (below − 253°C) for transportation.23 Hydrogen can also penetrate though materials when stored or transported through pipelines, making them brittle. Hydrogen gas has no smell, highly flammable and its flame is invisible to the naked eye. Safe handling of hydrogen is crucial to its mass deployment as an energy carrier. Thus, an alternative option is to convert low-carbon hydrogen to liquid organic hydrogen carriers such as methanol and ethanol, which are liquid at room temperature and can be easily transported within existing shipping networks. Alcohols could be used as fuels for high heat generation, and heavy transport, and could be converted to olefins and aromatics. Materials and process innovations converting intermittent renewable electricity to easily transportable chemicals as energy carriers, feedstocks, and long-term storage medium, possibly in a single step at a competitive cost, will be crucial for transitioning to a zero carbon economy.
Decarbonization plan for the chemical industry using low-carbon H2
We propose a multi-generational plan for decarbonization of the chemical industry, specifically for the energy-intensive processes such as ammonia, methanol, olefins, and aromatics, since the rest of the processes are mostly electricity-based and could be switched to low-carbon grid power once available. The current status of the amount of feedstocks, fuels, and raw materials and their emissions broken down by their source is depicted Figure 4a. The total energy need for the current processes is estimated as 12 exajoules (EJ) provided by fossil fuels, which is about 2% of the global energy demand. We assumed 50% growth of each raw material volume over 10–15 years (3–4% yearly growth) for each step of the decarbonization plan. In the next 10–15 years, coal as feedstock should be replaced with natural gas, methane emissions from feedstock and fuel production should be minimized, and all electricity use (2 EJ) should be procured from renewables (Figure 4b). This would keep total emissions at the same level while providing economic growth. The following 10–15 years, assuming low-carbon electricity and H2 would be available, any new volume growth in ammonia and methanol should be from low-carbon H2. Half of the new growth in olefins should be from electrified cracking and the other half should be from low-carbon H2 coupled with CO2 utilization (Figure 4c). We also propose that at least 30% of CO2 emissions from feedstock, fuel, ammonia, olefin, and aromatic production processes be captured and stored. This step would require an additional 23 EJ of renewable electricity, providing about 40% total emissions reduction while providing economic growth.
Multi-generational decarbonization plan for energy-intensive raw material processes in the chemical industry (see more details in the SI). In each part of the graph (a–d), blue bars without borders represent the amount of emissions from feedstock and fuels (on the left side) and raw materials (on the right side); gray and white bars with dark borders represent the amount of feedstocks and fuels consumed (left side of the arrow) and raw materials produced (right side of the arrows); yellow portion inside the gray bars represent the amount low-carbon version of that raw material; area of these bars are correlated to the amount of emissions and materials where scaling of the unit of 200 (blue) and 400 (gray) million tons are shown in the middle of the picture. Black and green bars above the arrows represent fossil-based and low-carbon primary energy needed for the production of feedstocks and their conversion to raw materials, where areas of the bars are correlated to the amount of EJ of energy written inside the bars. Boxes with dark borders and arrows between a–d parts of the figure summarize growth amount and measures taken to achieve the next state it points toward.
In the final 10–15 years, there should be a major decarbonization of the chemical industry, where any new volume in ammonia, methanol, and olefins and half of the aromatics would come from low-carbon H2 coupled with utilization of CO2 captured from chemical industry’s own emissions (Figure 4d). This step would require an additional 40 EJ of renewable electricity, bringing total energy use of the chemical industry to 79 EJ (65 EJ from renewables and 14 EJ from fossil fuels) while producing 3.5 times the current amounts of same raw materials. At this stage, the chemical industry would be nearly carbon neutral requiring about 305 million tons of low-carbon H2. The final case also assumes that plastic resins are recycled, not emitting more CO2 at the end of their lifetime. If current chemical processes went on without a decarbonization plan at a similar growth rate, they would require 42 EJ of fossil-based energy (53% of the alternative decarbonization case presented in Figure 4d), emitting 4.3 billion tons of CO2eq instead of 0.2 billion tons of CO2eq emitted in the final stage of the proposed decarbonization plan. Thus, avoiding 28 EJ of additional fossil energy use in this final stage requires the use of 65 EJ of renewable energy. This decarbonization plan would make the chemical industry a proportionally larger energy consumer per mass of raw materials produced.
Summary
Decarbonization of the power grid would enable the chemical industry to reduce its GHG emissions by nearly 35 percent. A handful of chemical processes (ammonia, methanol, olefins, aromatics) are the most difficult to decarbonize, accounting for half of the emissions from chemical industries. Low-carbon hydrogen production using water electrolysis followed by conversion to ammonia and methanol (possibly ethanol) could not only reduce another quarter of the emissions from chemicals but also act as a liquid storage option for hydrogen. The remaining olefins and aromatics could be made catalytically at lower temperatures running on renewable power. Materials innovation in membranes, sorbents and catalysts will be crucial to transitioning chemical production to a lower carbon future. Cost and availability of low-carbon electricity and hydrogen and changes in the policy regarding carbon trading and tax at certain geographies could affect the recommendations made in this article.
Data availability
All of the data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
O. Edenhofer, R. Pichs-Madruga, Y. Sokona, E. Farahani, S. Kadner, K. Seyboth, A. Adler, I. Baum, S. Brunner, P. Eickemeier, B. Kriemann, J. Savolainen, S. Schlömer, C. von Stechow, T. Zwickel, J.C. Minx, Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge, University Press, New York, 2014). https://www.ipcc.ch/report/ar5/wg3/
B. Bajželj, J.M. Allwood, J.M. Cullen, Designing climate change mitigation plans that add up. Environ. Sci. Technol. 14(47), 8062 (2013). https://doi.org/10.1021/es400399h
G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz, B. Weidema, The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life. Cycle Assess. 9(21), 1218 (2016). https://doi.org/10.1007/s11367-016-1087-8
S. Brueske, R. Sabouni, C. Zach, H. Andres, U.S. Manufacturing Energy Use and Greenhouse Gas Emissions Analysis (US Department of Energy, Washington, DC, 2012). https://www.energy.gov/sites/default/files/2013/11/f4/energy_use_and_loss_and_emissions.pdf
US Energy Information Administration (EIA), Levelized Costs of New Generation Resources in the Annual Energy Outlook 2021 (2021). https://www.eia.gov/outlooks/aeo/electricity_generation.php
International Renewable Energy Agency (IRENA), Renewable Power Generation Costs in 2020 (2021). https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2021/Jun/IRENA_Power_Generation_Costs_2020.pdf
R. Faiz, K. Li, Olefin/paraffin separation using membrane based facilitated transport/chemical absorption techniques. Chem. Eng. Sci. 73, 261 (2012). https://doi.org/10.1016/j.ces.2012.01.037
K. Adil, Y. Belmabkhout, R.S. Pillai, A. Cadiau, P.M. Bhatt, A.H. Assen, G. Maurin, M. Eddaoudi, Gas/vapour separation using ultra-microporous metal–organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 11(46), 3402 (2017). https://doi.org/10.1039/C7CS00153C
Y. Wang, S.B. Peh, D. Zhao, Alternatives to cryogenic distillation: Advanced porous materials in adsorptive light olefin/paraffin separations. Small 25(15), 1900058 (2019). https://doi.org/10.1002/smll.201900058
D.S. Sholl, R.P. Lively, Seven chemical separations to change the world. Nature 532, 435 (2016). https://doi.org/10.1038/532435a
H. Zimmermann, R. Walzl, Ethylene, in Ullmann’s Encyclopedia of Industrial Chemistry (Wiley-VCH, 2012). https://doi.org/10.1002/14356007.a10_045.pub3
T. Ren, M. Patel, K. Blok, Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 4(31), 425 (2006). https://doi.org/10.1016/j.energy.2005.04.001
A.M. Ward, S. Stevenson, A.J.M. Oprins, Z. Zhao, T. Abbott, K.F. Lawson, J.W. Schroer, M.E. Huckman, Use of renewable energy in olefin synthesis, European Patent EP3725865 A1 (2019). https://patents.google.com/patent/EP3725865A1/en?oq=EP3725865A1
J. Seppala, J. Hiltunen, V.-M. Purola, Process and rotary machine type reactor US Patent US2014/0243569A1, (2014). https://patents.google.com/patent/US20140243569A1/en?oq=US2014%2f0243569A1
H. Zimmermann, Propene, in Ullmann's Encyclopedia of Industrial Chemistry (Wiley-VCH, 2013). https://doi.org/10.1002/14356007.a22_211.pub3
M.P. Lapinski, S. Metro, P.R. Pujadó, M. Moser, Catalytic reforming in petroleum processing, in Handbook of Petroleum Processing. ed. by S.A. Treese, P.R. Pujadó, D.S.J. Jones (Springer, Cham, Switzerland, 2015), pp. 229–260. https://doi.org/10.1007/978-3-319-14529-7_1
S. Najari, S. Saeidi, P. Concepcion, D.D. Dionysiou, S.K. Bhargava, A.F. Lee, K. Wilson, Oxidative dehydrogenation of ethane: Catalytic and mechanistic aspects and future trends. Chem. Soc. Rev. 7(50), 4564 (2021). https://doi.org/10.1039/D0CS01518K
Y. Dai, X. Gao, Q. Wang, X. Wan, C. Zhou, Y. Yang, Recent progress in heterogeneous metal and metal oxide catalysts for direct dehydrogenation of ethane and propane. Chem. Soc. Rev. 9(50), 5590 (2021). https://doi.org/10.1039/D0CS01260B
M. Yang, D. Fan, Y. Wei, P. Tian, Z. Liu, Recent progress in methanol-to-olefins (MTO) catalysts. Adv. Mater. 50(31), 1902181 (2019). https://doi.org/10.1002/adma.201902181
D. Gao, X. Qiu, Y. Zhang, P. Liu, Life cycle analysis of coal based methanol-to-olefins processes in China. Comput. Chem. Eng. 109, 112 (2018). https://doi.org/10.1016/j.compchemeng.2017.11.001
A.M. Bazzanella, F. Ausfelder, Low carbon energy and feedstock for the european chemical industry (DECHEMA, Frankfurt, Germany, 2017). https://dechema.de/dechema_media/Downloads/Positionspapiere/Technology_study_Low_carbon_energy_and_feedstock_for_the_European_chemical_industry.pdf
G.A. Olah, A. Goeppert, G.K.S. Prakash, Beyond Oil and Gas: The Methanol Economy (Wiley-VCH, Weinheim, Germany, 2009)
International Energy Agency (IEA), The Future of Hydrogen: Seizing Today's Opportunities (2019). https://www.iea.org/reports/the-future-of-hydrogen
International Renewable Energy Agency (IRENA), Green Hydrogen Cost Reduction: Scaling up Electrolysers to Meet the 1.5°C Climate Goal (2020). https://irena.org/-/media/Files/IRENA/Agency/Publication/2020/Dec/IRENA_Green_hydrogen_cost_2020.pdf
International Energy Agency (IEA), Net Zero by 2050: A Roadmap for the Global Energy Sector (2021). https://www.iea.org/reports/net-zero-by-2050
Hydrogen Council, Hydrogen decarbonization pathways: A life cycle assessment, (2021). https://hydrogencouncil.com/wp-content/uploads/2021/01/Hydrogen-Council-Report_Decarbonization-Pathways_Part-1-Lifecycle-Assessment.pdf
Z. Yan, J.L. Hitt, J.A. Turner, T.E. Mallouk, Renewable electricity storage using electrolysis. Proc. Natl. Acad. Sci. U.S.A. 23(117), 12558 (2020). https://doi.org/10.1073/pnas.1821686116
A. Kätelhön, R. Meys, S. Deutz, S. Suh, A. Bardow, Climate change mitigation potential of carbon capture and utilization in the chemical industry. Proc. Natl. Acad. Sci. U.S.A. 23(116), 11187 (2019). https://doi.org/10.1073/pnas.1821029116
S.A. Stevenson, Thermodynamic considerations in CO2 utilization. AIChE J. 9(65), e16695 (2019). https://doi.org/10.1002/aic.16695
N. Sánchez-Bastardo, R. Schlögl, H. Ruland, Methane pyrolysis for CO2-free H2 production: A green process to overcome renewable energies unsteadiness. Chem. Ing. Tech. 10(92), 1596 (2020). https://doi.org/10.1002/cite.202000029
N. von der Assen, L.J. Müller, A. Steingrube, P. Voll, A. Bardow, Selecting CO2 sources for CO2 utilization by environmental-merit-order curves. Environ. Sci. Technol. 3(50), 1093 (2016). https://doi.org/10.1021/acs.est.5b03474
E.S. Sanz-Pérez, C.R. Murdock, S.A. Didas, C.W. Jones, Direct capture of CO2 from ambient air. Chem. Rev. 19(116), 11840 (2016). https://doi.org/10.1021/acs.chemrev.6b00173
Acknowledgements
Authors are employed by The Dow Chemical Company. No other funding sources are used for generation of the results and creation of the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eryazici, I., Ramesh, N. & Villa, C. Electrification of the chemical industry—materials innovations for a lower carbon future. MRS Bulletin 46, 1197–1204 (2021). https://doi.org/10.1557/s43577-021-00243-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43577-021-00243-9