Abstract
Background
Pharmacotherapies remain a central focus of successful tobacco control, but uptake remains very low.
Objective
To estimate the cost effectiveness of a primary care nicotine replacement therapy (NRT) sampling intervention.
Design
A Markov cohort simulation model was constructed to conduct cost-effectiveness analyses. Clinical trial results were used to initialize the Markov model. All other model parameters were derived from the literature. The study was conducted over a lifetime horizon, from the payers’ budgetary perspective.
Participants
Smokers with a primary care visit.
Intervention
Medication sampling, which provided short, starter packets of NRT (nicotine patch and lozenge) to smokers in the primary care setting.
Main Measures
Lifetime healthcare expenditures, quality-adjusted life years, and life years.
Key Results
Medication sampling was the dominant strategy compared to standard care. Our intervention cost $75, yielding a discounted lifetime savings of $1065 in healthcare expenditures, and increased both discounted quality-adjusted life years and discounted life years by 0.01. One-way sensitivity analyses showed that medication sampling remained dominant in plausible ranges except when it failed to increase cessation relative to standard care. Probabilistic sensitivity analyses confirmed that medication sampling was dominant in 94.1% of the simulated cases, with an implementation cost of $74 (95% CI $73–$76) and discounted lifetime savings in health expenditures of $1061 (− $1106 to − $1,017), increasing quality-adjusted life years by 0.008 (0.0085–0.0093) and life years by 0.008 (0.0081–0.0089).
Conclusion
Medication sampling, an easily implementable, scalable and low-cost intervention to encourage smoking cessation, is cost saving and improves quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Pharmacotherapies continue to be a central focus of clinical efforts toward successful tobacco control. Seminal reviews1,2,3,4,5 and recommendation statements6,7,8 consistently suggest that (1) all tobacco users should be offered brief advice to assist with quitting, and (2) unless medically contraindicated, all smokers should be offered pharmacotherapy. Unfortunately, uptake of cessation pharmacotherapies remains very low.9,10,11,12 Only about half of all smokers make a quit attempt in any given year,13 and less than 25% of these quit attempters use pharmacotherapy.9,14 Clearly, there is a strong need to identify new methods to get more smokers to use better treatments.
Medication sampling refers to providing short, starter packets of nicotine replacement therapy (NRT), either as a stand-alone regimen or added to other treatment protocols. The intent is to engage smokers in the process of quitting, regardless of their intention to quit, and without any requirement or expectation to quit immediately. Within the sampling exercise, instructions are kept minimal to increase scalable potential for provision within clinical settings. We previously tested medication sampling in four separate trials,15,16,17,18 one of which was implemented within primary care settings as a hybrid efficacy/effectiveness trial.16,19 Results from these trials were generally positive, yielding clinically and statistically significant improvements in cessation.
Methods19 and outcomes16,20,21 of these trials have been reported elsewhere. Briefly, within a cluster-RCT, we enrolled 1245 smokers across the motivational spectrum, across 22 primary care clinics throughout South Carolina between 2014 and 2018. All interventions were delivered directly by healthcare providers during routine clinic visits. In the “standard care” arm, providers gave a bag to all participants, including standard smoking cessation information and brochures for the state Quitline. For the “NRT” arm, a 2-week supply of nicotine patch (14 mg) and lozenge (4 mg) was added to the standard care package. Brief advice was given to all patients, and all providers were trained in the 5As (ask, advise, assess, assist, and arrange) at study initiation. There was no other intervention provided to either group. The trial showed that 6-month abstinence rates of NRT sampling and standard care are 12% and 8%, respectively.16
An enduring question about medication sampling is whether this strategy confers any long-term cost effectiveness. On one hand, the intervention is inexpensive and easy to deliver—especially in primary care, where 70% of all smokers visit at least once annually.22,23 NRT sampling does not require extensive primary care involvement, and explanation of rationale to both smoker and provider is face valid. On the other hand, absolute rates of quitting are low. Moreover, many smokers who are given medication samples will not use them at all (~ 40% in our trial), resulting in a waste of resources.
Thus, the “return on investment” of medication sampling is unclear. Simple estimations from our primary care trial suggest a cost per quit ~ $475, similar to or lower than Quitline-based medication give-away programs.24,25,26,27 However, any real-world application of medication sampling will depend on an in-depth assessment of cost effectiveness based on health outcomes that take years to materialize. Such an assessment should also provide information on the population-level effect of large-scale NRT sampling, as other interventions may have similar cost per quit attempt but may not be scalable. Simulation modeling, which we propose to undertake herein, is the only feasible method to calculate the cost effectiveness of an intervention with distal outcomes that take years for results to materialize.
METHODS
Markov Cohort Model Overview
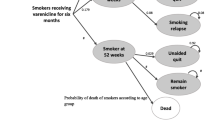
Our approach is based on a Markov cohort model that simulates smoking behaviors over a lifetime. We took the structure of the model that was developed by Barnett et al.28 to accrue lifetime healthcare expenditures, quality-adjusted life years (QALYs), and life years (LYs) to conduct our cost-effectiveness analyses. The Markov cohort model consists of only three health states, “current smoker,” “former smoker,” and “dead” (Fig. 1). In each simulation cycle of 3 months, current smokers may quit, die, or continue to smoke, and former smokers may remain abstinent, relapse, or die. All transitions between health states are based on age-, sex-, and smoking status–specific mortality probabilities and smoking status–specific quit and relapse probabilities. Discounted costs, QALYs, and LYs over the lifetime are accrued based on projected lengths of time spent in each health state and the average healthcare expenditures associated with each health state. We obtained age, sex, and smoking status of the simulation cohorts and NRT sampling costs from our trial (Appendix Table 1), but all other model parameters were derived from the literature, as described in Barnett et al.28 For all literature-derived parameters, we adopted the underlying general population parameters unadjusted for a psychiatric patient population in the Barnett study. (See Appendix Tables 2–4 for key model parameters.)
Cost of NRT Sampling
The 2-week cost of NRT sampling was estimated to be $75. While the intervention consisted of both provider time and costs for cessation brochures, these added costs are not included because only the NRT sample differed across treatment groups. However, in subsequent one-way and probabilistic sensitivity analyses, we vary the intervention cost between $37.50 and $112.50, where the higher ranges can cover additional labor costs, or more costly or longer-duration medications.
Mortality Rates
We derived sex- and age-specific mortality rates for the general population directly from the 2017 Social Security Administration’s Actuarial Life Table.29 We then applied excess death hazards to estimate the mortality rates for smokers based on age and sex. These smoking-related excess mortality hazards vary by sex and three broad age brackets (24–54 years, 55–74 years, and 75+ years),30,31,32,33,34,35,36,37 but the underlying mortality rates differ by sex and 5-year age increments. Excess death hazards fall among former smokers after 5 years of abstinence.
Healthcare Expenditures
We obtained age- and sex-specific average annual healthcare expenditures from the Medical Care Expenditures Panel Survey (MEPS).38 We updated the latest available figures (from 2014) to 2019 expenditures using the cost inflation index from the Centers for Medicare and Medicaid Services’ National Health Expenditures Fact Sheet.39 We then multiplied these estimated age- and sex-specific expenditures by ratios representing how much more smokers and former smokers spend on healthcare versus non-smokers. These ratios were derived from healthcare charges in large employer’s health plans,40 comparing healthcare spending among current smokers, recent quitters (< 5 years), and long-term quitters (5+ years). Following patterns observed in the literature,41,42,43 we assume that former smokers have elevated healthcare spending around the time of cessation, but then experience a sustained reduction in spending after 5 years of abstinence.
QALY Weights, Natural Quit Rates, and Relapse Rates
We used age-, sex-, and smoking status–specific QALY weights derived from the literature to adjust total life years lived based on each cohort’s distribution of sex, age, and smoking status.44 We made the simplifying assumption that quality of life among former smokers does not depend on length of time since quitting.28 Smoking status varies over time according to the natural background successful quit attempt rates and relapse rates, which are determined by the length of abstinence.45,46,47,48 We used an annual natural quit rate of 4.3%.49,50 We adopted relapse rates of 60% within 1 year of quitting, which falls to 47% in year 2, 4% in years 3–5, 2% in years 6–9, and 1% after 10 or more years.48,51 We specifically chose high initial relapse rates that fall sharply after two full years of abstinence,48 given that a one-time, non-intensive intervention may not hold in the short run.
Discounting
All costs and outcomes were discounted at 3% per year. Costs were denominated in 2019 US Dollars. We used $100,000 per QALY as the threshold for willingness to pay.52
Cost-Effectiveness Analyses
We conducted the base-case cost-effectiveness analysis by running the simulation with one branch for NRT sampling and one for standard care. All model parameters were identical between the two cohorts except the initial distribution of former versus current smokers. Each cohort was 30.1% male, with everyone aged 50.72 years based on the mean age from our trial results. The model projected the cost and health outcome outputs for the two arms over the lifetime, from which we calculated the incremental cost (NRT samples) and the incremental benefit (savings in healthcare expenditures, QALY, and LY) of NRT sampling versus standard care.
One-Way Sensitivity Analyses and Probabilistic Sensitivity Analyses
We first conducted one-way sensitivity analyses, varying one parameter value at a time to assess model robustness to parameter changes. We then conducted a probabilistic sensitivity analysis (varying all parameters simultaneously), using 1000 independent random draws from each parameter’s distributions. We drew costs from a gamma distribution, mortality hazard ratios from a triangular distribution, and QALYs and all probabilities from beta distributions. These distributions were set to reflect ranges or confidence intervals of the respective parameters. (See Appendix Tables 2, 3 and 4). Using these 1000 incremental cost-effectiveness pairs between NRT sampling and standard care, we generated the “confidence interval” for our baseline cost-effectiveness results, taking into account the uncertainty of parameter values.
Counterfactual Analyses
As currently conceived, patients only receive a one-time sample of medication. Counterfactual scenarios allow us to model options for recurrent sampling. We conducted two counterfactual analyses, in which 50% of the participants who remained smokers could randomly receive another NRT sample in each quarter of the first 6 months (counterfactual A) or first 12 months (counterfactual B) after model initiation. We assumed, as a base-case scenario, that multiple NRT samples would have the same quit rates as the initial sample.
We constructed the model using TreeAge Pro 2021 (Williamstown, MA). Additional details on the model can be found in the Supplemental Appendix.
RESULTS
Baseline Characteristics
The clinic19 and participant16 characteristics have been described elsewhere; baseline participant characteristics for the Markov model are presented in Appendix Table 1. Six-month quit rates, within both NRT sampling (12%) and standard care arms (8%), represented the primary model inputs for our simulation model.
Cost-Effectiveness Findings
The results of the base-case model are presented in Table 1. Discounted lifetime follow-up healthcare expenditures for NRT sampling were $322,939, versus $324,079 for standard care. NRT sampling cost an additional $75 per person to implement, but resulted in a net savings of $1065 per person over a lifetime. The cohort receiving NRT sampling was projected to live on average 16.609 life years, or 0.01 life year more than the 16.599 life years realized under standard care. NRT sampling yielded 12.861 quality-adjusted life years (QALYs), or 0.01 QALY more than the 12.851 QALYs under standard care. Therefore, NRT sampling is a dominant strategy because it yields higher lifetime QALYs at a lower lifetime cost.
One-Way Sensitivity Analyses
We considered many parameters, including effect sizes between 0 and 8% and time-dependent relapse rates ranging from 50 to 150% of the baseline relapse rates.48,51 These results, presented in Table 2, show that NRT sampling is the dominant strategy in all but one scenario in which NRT sampling provides no additional successful quits. We also conducted a threshold analysis and found that NRT sampling need only achieve an increase of 0.262% in abstinence relative to standard care to justify the NRT costs.
Probabilistic Sensitivity Analysis
Table 1 (right panel) provides the confidence intervals around the simulated costs and outcomes, as well as around the difference in costs and outcomes between NRT sampling and standard care. The results show that relative to standard care, NRT sampling decreases costs by $1061 (95% CI $1017–$1106) and increases discounted life years by 0.008 (95% CI 0.0081–0.0089) and discounted QALYs by 0.008 (95% CI 0.0085–0.0093). In Appendix Figure 2, we present an incremental cost-effectiveness scatterplot, showing that > 94.1% of these 1000 pairs are cost saving at the willingness-to-pay threshold of $100,000 per QALY gained.
Counterfactual Analyses
In Table 3, we show that from $1065 in net savings for a one-time administration of NRT sampling, the extended model projected a net savings of $1275 and $1412 in the counterfactual analyses, extending the NRT sampling period to 6 months and 12 months, respectively. QALYs gained also increased from 0.008 in the original one-shot model, to 0.013 and 0.015 in the two extended counterfactual models.
DISCUSSION
Our model shows that NRT sampling increases lifetime QALYs and decreases lifetime costs relative to standard care in the great majority of scenarios. If we ignore the lifetime reduction in healthcare expenditures associated with sustained quitting and consider only the cost of implementation and QALY gains, the incremental cost per QALY was approximately $7500, which is considered cost effective. Given the totality of findings here, NRT sampling achieves robust results in cost savings and improves quality of life, even when overall quit rates are low.
The QALY improvements and cost savings may at first glance appear to be modest. It is therefore important to place these figures in context. First, our study results appear favorable in terms of cost per quit for tobacco cessation interventions. A recent meta-analysis53 suggests that the average cost per quit for pharmacological interventions was approximately $19,510.54,55 Behavioral interventions cost on average $11,416 per quit,27,56,57,58,59,60 and combined behavioral and pharmacological interventions were on average $14,662 per quit.53 NRT sampling, however, costs approximately $475 per quit. Moreover, a comprehensive assessment of 380 public health interventions between 2005 and 2018 by the UK’s National Institute for Health and Care Excellence revealed that the median cost per QALY gained was £1986 ($2641 US Dollars), with 21% of interventions found to be cost saving.61 In other words, our cost-saving NRT sampling is in the top 20% of all public health interventions, not just tobacco cessation interventions.
Moreover, NRT sampling is feasible and scalable because of its ease of implementation. Other interventions may need to recruit participants, provide a longer-term cessation program, and/or add a high-cost cognitive behavioral component.56 Numerous studies have evaluated the effectiveness of pharmacological interventions around the world in multiple settings,27,54,55,60,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77 ranging from $426 per QALY (telemedicine)59,78 to $340,000 per QALY (school-based education).79 Many of these studies were based on more intensive interventions and achieved higher quit rates than did NRT sampling herein. However, as a brief (~ 1 min) and pragmatic intervention, medication sampling offers significant scalability, particularly within primary care. Thus, low quit rates may be offset by high reach.
The results of our simulation are insensitive to manipulation of model parameters across plausible ranges. What drove our model results was simply differences in the initial distribution of current and former smokers. Any intervention that is sufficiently low cost, increases the quit rate, and implementable on a large scale can generate positive savings over a lifetime horizon. Based on our simulation results, a health plan that chooses to spend $75,000 ($75 * 1000) in NRT sampling on 1000 current smokers can on average expect to recover $1,140,000 in averted healthcare spending over the lifetime, if enrollees remain in the same health plan.
We also show that if subsequent reissues of NRT sampling retain the same rate of cessation as our original trial, the cost savings will increase with each re-issue. We acknowledge, however, that it is unclear whether repeated administrations of NRT sampling will yield similar quit rates. Theoretically, an intervention that failed the first time may not succeed on a second attempt. On the other hand, tobacco cessation often requires multiple attempts to be successful.80 Future research should consider examining the effect of multiple or different NRT samples.
Finally, higher cost and higher effect strategies will continue to drive policy efforts to reduce the burden of smoking. However, approaches such as NRT sampling, representing low-cost, modest-effect, high-reach interventions that are feasible and scalable, also have a place in the policy armamentarium to encourage smoking cessation. Such programs can also be administered in conjunction with established evidence-based tobacco treatment interventions.
A key question is to understand how durable the abstinence rates remain after NRT sampling. As shown by our one-way sensitivity analyses, the higher the relapse rate, the less cost saving NRT sampling becomes. At 50% of the relapse rates, net savings from NRT sampling reach $2220 per person, but at 150% of the base-case analysis, net savings fall dramatically to only $201. In fact, relapse rates of over 90% in both year 1 and year 2 will drop savings in subsequent healthcare spending so low that they will be less than the $75 cost of NRT. Future research should consider developing and testing additional pragmatic interventions to analyze and, if necessary, reduce relapse rates after the initial NRT sampling.
LIMITATIONS
Our model structure focused on population averages, with very little accommodation for established clinical and sociodemographic differences that may affect outcomes. Future modeling efforts should include other patient characteristics to model that are important to tailor and design policy. Abstinence rates from our study were also not biochemically verified, although there are plans to do so in future studies. In addition, future modeling efforts may also consider disaggregating health states into major smoking–related illnesses. Finally, many of the model parameters are drawn from studies that may be dated today. As new evidence is accumulated, future modeling should incorporate the latest information on how smoking affects health.
CONCLUSION
NRT sampling is cost saving ($1065 in savings per person) when we take into consideration reduced health expenditures over a lifetime for those who quit. When we ignore these lifetime cost reductions, NRT sampling costs approximately $475 per quit and $7500 per QALY gained. These figures are well within the acceptable range of $100,000 per QALY gained. While interventions with higher effectiveness exist, they are costlier to implement, and many are difficult to scale to a large population. NRT sampling should therefore be considered an appropriate and useful addition to the existing policy toolkit to reduce the mortality and morbidity burden of smoking.
References
Zhu SH, Lee M, Zhuang YL, Gamst A, Wolfson T. Interventions to increase smoking cessation at the population level: how much progress has been made in the last two decades? Tobacco Control. 2012;21:110-8.
Cox LS, Okuyemi K, Choi WS, Ahluwalia JS. A review of tobacco use treatments in U.S. ethnic minority populations. American Journal of Health Promotion. 2011;25(5 Suppl):S11-30.
Carpenter MJ, Ford ME, Cartmell KB, Alberg AJ. Misperceptions and misconceptions of nicotine replacement therapy within racially and ethnically diverse smokers. Journal of the National Medical Association. 2011;103:885-94.
Cummings KM, Hyland A. Impact of nicotine replacement therapy on smoking behavior. Annual Review of Public Health. 2005;26:583-99.
Hughes JR, Shiffman S, Callas P, Zhang J. A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tobacco Control. 2003;12:21-7.
Winchell C, Raffaelli RM, Roca R, Michele T. Food and Drug Administration response to the ATTUD/SRNT policy statement on the labeling of nicotine replacement therapies. Nicotine & Tobacco Research. 2016;18:1218-9.
Fucito LM, Bars MP, Forray A, Rojewski AM, Shiffman S, Selby P, et al. Addressing the evidence for FDA nicotine replacement therapy label changes: A policy statement of the Association for the Treatment of Tobacco use and Dependence and the Society for Research on Nicotine and Tobacco. Nicotine and Tobacco Research. 2014;16:909-14.
Toll BA, Brandon TH, Gritz ER, Warren GW, Herbst RS, AACR Subcommittee on Tobacco and Cancer. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clinical Cancer Research. 2013;19:1941-8.
Dahne J, Wahlquist AE, Garrett-Mayer E, Heckman BW, Cummings KM, Carpenter MJ. State tobacco policies as predictors of evidence-based cessation method usage: Results from a large, nationally representative dataset. Nicotine & Tobacco Research. 2018;20:1336-43.
Pacek LR, McClernon FJ, Bosworth HB. Adherence to pharmacological smoking cessation interventions: A literature review and synthesis of correlates and barriers. Nicotine & Tobacco Research. 2018;20:1163-72.
Black A, Beard E, Brown J, Fidler J, West R. Beliefs about the harms of long-term use of nicotine replacement therapy: Perceptions of smokers in England. Addiction. 2012;11:2037-42.
Fix BV, Hyland A, Rivard C, McNeill A, Fong GT, Borland R, et al. Usage patterns of stop smoking medications in Australia, Canada, the United Kingdom, and the United States: Findings from the 2006-2008 International Tobacco Control (ITC) Four Country Survey. International Journal of Environmental Research and Public Health. 2011;8(1):222-33.
USDHS. Quitting Smoking Among Adults — United States, 2000–2015. MMWR. 2017;65:1457-64.
Dahne J, Wahlquist AE, Garrett-Mayer E, Heckman BW, Cummings KM, Carpenter MJ. The differential impact of state tobacco control policies on cessation treatment utilization across established tobacco disparities groups. Preventive Medicine. 2017;105:319-25.
Carpenter MJ, Gray KM, Wahlquist AE, Cropsey K, Saladin ME, Froeliger B, et al. A pilot randomized clinical trial of remote varenicline sampling to promote treatment engagement and smoking cessation. Nicotine and Tobacco Research. 2021;23(6):983-91.
Carpenter MJ, Wahlquist AE, Dahne J, Gray KM, Garrett-Mayer E, Cummings KM, et al. Nicotine replacement therapy sampling for smoking cessation within primary care: Results from a pragmatic cluster randomized clinical trial. Addiction. 2020;115:1358-67.
Jardin BF, Cropsey KL, Wahlquist AE, Gray KM, Silvestri GA, Cummings KM, et al. Evaluating the effect of access to free medication to quit smoking: A clinical trial testing the role of motivation. Nicotine & Tobacco Research. 2014;16:992-9.
Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, Alberg AJ. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: A randomized clinical trial. Archives of Internal Medicine. 2011;171:1901-7.
Dahne J, Wahlquist AE, Boatright AS, Garrett-Mayer E, Fleming DO, Davis R, et al. Nicotine replacement therapy sampling via primary care: Methods from a pragmatic cluster randomized clinical trial. Contemporary Clinical Trials. 2018;72:1-7.
Silvestri NJ, Dahne J, Wahlquist AE, Toll B, Carpenter MJ. Does medication sampling improve compliance with brief advice? Results from a pragmatic randomized clinical trial. Journal of Smoking Cessation. 2021;2021:1-4.
Dahne J, Wahlquist AE, Smith TT, Carpenter MJ. The differential impact of nicotine replacement therapy sampling on cessation outcomes across established tobacco disparities groups. Preventive Medicine. 2020;136:106096.
Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting Smoking Among Adults - United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-64.
Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services. 2008.
Fellows JL, Bush T, McAfee T, Dickerson J. Cost effectiveness of the Oregon quitline "free patch initiative". Tobacco Control. 2007;16(Suppl 1):i47-i52.
Krupski L, Cummings KM, Hyland A, Mahoney MC, Toll BA, Carpenter MJ, et al. Cost and effectiveness of combination nicotine replacement therapy among heavy smokers contacting a quitline. Journal of Smoking Cessation. 2014:11(1):50-9.
Cummings KM, Hyland A, Carlin-Menter S, Mahoney MC, Willett J, Juster HR. Costs of giving out free nicotine patches through a telephone quit line. Journal of Public Health Management and Practice. 2011;17(3):E16-23.
Cummings KM, Fix B, Celestino P, Carlin-Menter S, O'Connor R, Hyland A. Reach, efficacy, and cost-effectiveness of free nicotine medication giveaway programs. Journal of Public Health Management Practice. 2006;12:37-43.
Barnett PG, Wong W, Jeffers A, Hall SM, Prochaska JJ.Cost-effectiveness of smoking cessation treatment initiated during psychiatric hospitalization: analysis from a randomized, controlled trial. J Clin Psychiatry. 2015;76(10):e1285-91.
Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey. Retrieved June 10, 2021, from https://www.meps.ahrq.gov/mepsweb/.
Friedman GD, Tekawa I, Salder M, Sidney S. Smoking and mortality: the Kaiser Permanente experience. Changes in cigarette-related disease risks and their implications for prevention and control. Monogr. Natl. Cancer Inst. 1997;97:472-99.
LaCroix AZ, Lang J, Scherr P, Wallace RB, Cornoni-Huntley J, Berkman L, et al. Smoking and mortality among older men and women in three communities. N Engl J Med. 1991;324(23):1619-25.
Taylor DH, Jr., Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. Am J Public Health. 2002;92(6):990-6.
van Baal PH, Hoogenveen RT, de Wit GA, Boshuizen HC. Estimating health-adjusted life expectancy conditional on risk factors: results for smoking and obesity. Popul Health Metr. 2006;4:14.
Prescott E, Osler M, Andersen PK, Hein HO, Borch-Johnsen K, Lange P, et al. Mortality in women and men in relation to smoking. Int J Epidemiol. 1998;27(1):27-32.
Sloan FA. The price of smoking. Cambridge, Mass.: MIT Press; 2004.
Woloshin S, Schwartz LM, Welch HG. The risk of death by age, sex, and smoking status in the United States: putting health risks in context. J Natl Cancer Inst. 2008;100(12):845-53.
Woloshin S, Schwartz LM, Welch HG. Risk charts: putting cancer in context. J Natl Cancer Inst. 2002;94(11):799-804.
Agency for Healthcare Research and Quality 2014;Pages https://www.meps.ahrq.gov/mepsweb/ on June 10 2021.
Centers for Disease Control and Prevention. 2019 National Health Expenditures Fact Sheet; 2021. Retrieved April 24, 2021, from https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.
Musich S, Faruzzi SD, Lu C, McDonald T, Hirschland D, Edington DW. Pattern of Medical Charges after Quitting Smoking among Those with and without Arthritis, Allergies, or Back Pain. American Journal of Health Promotion. 2003;18(2):133-42.
Fishman PA, Khan ZM, Thompson EE, Curry SJ. Health Care Costs among Smokers, Former Smokers, and Never Smokers in an HMO. Health Services Research. 2003;38(2):733-49.
Fishman PA, Thompson EE, Merikle E, Curry SJ. Changes in Health Care Costs Before and After Smoking Cessation. Nicotine & Tobacco Research. 2006;8(3):393-401.
Hockenberry JM, Curry SJ, Fishman PA, Baker TB, Fraser DL, Cisler RA, et al. Healthcare costs around the time of smoking cessation. American Journal of Preventive Medicine. 2012;42(6):596-601.
Vogl M, Wenig CM, Leidl R, Pokhrel S. Smoking and health-related quality of life in English general population: implications for economic evaluations. BMC Public Health. 2012;12(1):203.
Hawkins J, Hollingworth W, Campbell R.Long-Term Smoking Relapse: A Study Using the British Household Panel Survey. Nicotine & Tobacco Research. 2010;12(12):1228-35.
Gilpin EA, Pierce JP, Farkas AJ, Farkas AJ. Duration of Smoking Abstinence and Success in Quitting. JNCI: Journal of the National Cancer Institute. 1997;89(8):572.
Wetter DW, Cofta-Gunn L, Fouladi RT, Cinciripini PM, Sui D, Gritz ER. Late relapse/sustained abstinence among former smokers: a longitudinal study. Preventive Medicine. 2004;39(6):1156-63.
Krall EA, Garvey AJ, Garcia RI. Smoking relapse after 2 years of abstinence: findings from the VA Normative Aging Study. Nicotine & Tobacco Research. 2002;4(1):95-100.
Levy DT, Graham AL, Mabry PL, Abrams DB, Orleans CT. Modeling the impact of smoking-cessation treatment policies on quit rates. Am J Prev Med. 2010;38(3 Suppl):S364-72.
Hyland A, Li Q, Bauer JE, Giovino GA, Steger C, Cummings KM. Predictors of cessation in a cohort of current and former smokers followed over 13 years. Nicotine Tob Res. 2004;6 Suppl 3:S363-9.
García-Rodríguez O, Secades-Villa R, Flórez-Salamanca L, Okuda M, Liu S-M, Blanco C. Probability and predictors of relapse to smoking: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug and Alcohol Dependence. 2013;132(3):479-85.
Cameron D, Ubels J, Norström F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Global Health Action. 2018;11(1):1447828.
Ali A, Kaplan CM, Derefinko KJ, Klesges RC. Smoking Cessation for Smokers Not Ready to Quit: Meta-analysis and Cost-effectivenessAnalysis. American Journal of Preventive Medicine. 2018;55(2):253-62.
Ebbert JO, Hughes JR, West RJ, Rennard SI, Russ C, McRae TD, et al. Effect of Varenicline on Smoking Cessation Through Smoking Reduction: A Randomized Clinical Trial. JAMA. 2015;313(7):687-94.
Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerstrom KO. Efficacy of Varenicline to Prompt Quit Attempts in Smokers Not Currently Trying to Quit: A Randomized Placebo-Controlled Trial. Nicotine & Tobacco Research. 2011;13(10):955-64.
Barnett PG, Wong W, Jeffers A, Munoz R, Humfleet G, Hall S.Cost-effectiveness of extended cessation treatment for older smokers. Addiction. 2014;109(2):314-22.
Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tobacco Control. 2007;16(Suppl 1):i53-i9.
McAlister AL, Rabius V, Geiger A, Glynn TJ, Huang P, Todd R. Telephone assistance for smoking cessation: one year cost effectiveness estimations. Tobacco Control. 2004;13(1):85-6.
Richter KP, Shireman TI, Ellerbeck EF, Cupertino AP, Catley D, Cox LS, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. J Med Internet Res. 2015;17(5):e113.
Ruger JP, Weinstein MC, Hammond SK, Kearney MH, Emmons KM.Cost-effectiveness of motivational interviewing for smoking cessation and relapse prevention among low-income pregnant women: a randomized controlled trial. Value Health. 2008;11(2):191-8.
Owen L, Fischer A. The cost-effectiveness of public health interventions examined by the National Institute for Health and Care Excellence from 2005 to 2018. Public Health. 2019;169:151-62.
Annemans L, Nackaerts K, Bartsch P, Prignot J, Marbaix S. Cost Effectiveness of Varenicline in Belgium, Compared with Bupropion, Nicotine Replacement Therapy, Brief Counselling and Unaided Smoking Cessation. Clinical Drug Investigation. 2009;29(10):655-65.
Athanasakis K, Igoumenidis M, Karampli E, Vitsou E, Sykara G, Kyriopoulos J.Cost-Effectiveness of Varenicline Versus Bupropion, Nicotine-Replacement Therapy, and Unaided Cessation in Greece. Clinical Therapeutics. 2012;34(8):1803-14.
Bae JY, Kim CH, Lee EK. Evaluation of Cost-Utility of Varenicline Compared with Existing Smoking Cessation Therapies in South Korea. Value in Health. 2009;12(s3):S70-S3.
Baker CL, Pietri G. A cost-effectiveness analysis of varenicline for smoking cessation using data from the EAGLES trial. ClinicoEconomics and Outcomes Research: CEOR. 2018;10:67.
Barnett PG, Ignacio RV, Kim HM, Geraci MC, Essenmacher CA, Hall SV, et al. Cost-effectiveness of real-world administration of tobacco pharmacotherapy in the United States Veterans Health Administration. Addiction. 2019;114(8):1436-45.
Bolin K, Wilson K, Benhaddi H, de Nigris E, Marbaix S, Mork A-C, et al. Cost-effectiveness of varenicline compared with nicotine patches for smoking cessation—results from four European countries. European Journal of Public Health. 2009;19(6):650-4.
Fellows JL, Bush T, McAfee T, Dickerson J. Cost effectiveness of the Oregon quitline “free patch initiative”. Tobacco Control. 2007;16(Suppl 1):i47-i52.
Graham AL, Chang Y, Fang Y, Cobb NK, Tinkelman DS, Niaura RS, et al. Cost-effectiveness of internet and telephone treatment for smoking cessation: an economic evaluation of The iQUITT Study. Tobacco Control. 2013;22(6):e11-e.
Hoogendoorn M, Welsing P, Rutten-van Mölken MP.Cost-effectiveness of varenicline compared with bupropion, NRT, and nortriptyline for smoking cessation in the Netherlands. Current Medical Research and Opinion. 2008;24(1):51-61.
Howard P, Knight C, Boler A, Baker C. Cost-Utility Analysis of Varenicline versus Existing Smoking Cessation Strategies using the BENESCO Simulation Model. J PharmacoEconomics. 2008;26(6):497-511.
Maciosek MV, LaFrance AB, Dehmer SP, McGree DA, Xu Z, Flottemesch TJ, et al. Health Benefits and Cost-Effectiveness of Brief Clinician Tobacco Counseling for Youth and Adults. The Annals of Family Medicine. 2017;15(1):37-47.
Ward CE, Hall SV, Barnett PG, Jordan N, Duffy SA.Cost-effectiveness of a nurse-delivered, inpatient smoking cessation intervention. Translational Behavioral Medicine. 2019;10(6):1481-90.
Altman D, Clement FM, Barnieh L, Manns B, Penz E. Cost-effectiveness of universally funding smoking cessation pharmacotherapy. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine. 2019;3(2):67-75.
MacMonegle AJ, Nonnemaker J, Duke JC, Farrelly MC, Zhao X, Delahanty JC, et al. Cost-Effectiveness Analysis of The Real Cost Campaign's Effect on Smoking Prevention. American Journal of Preventive Medicine. 2018;55(3):319-25.
Cadham CJ, Cao P, Jayasekera J, Taylor KL, Levy DT, Jeon J, et al. Cost-Effectiveness of Smoking Cessation Interventions in the Lung Cancer Screening Setting: A Simulation Study. JNCI: Journal of the National Cancer Institute. 2021;113(8):1065-73.
González-Roz A, Weidberg S, García-Pérez Á, Martínez-Loredo V, Secades-Villa R, Group ftABR.One-Year Efficacy and Incremental Cost-effectiveness of Contingency Management for Cigarette Smokers With Depression. Nicotine & Tobacco Research. 2020;23(2):320-6.
Daly AT, Deshmukh AA, Vidrine DJ, Prokhorov AV, Frank SG, Tahay PD, et al. Cost-effectiveness analysis of smoking cessation interventions using cell phones in a low-income population. Tobacco Control. 2019;28(1):88-94.
Tengs TO, Osgood ND, Chen LL. The Cost-Effectiveness of Intensive National School-Based Anti-Tobacco Education: Results from the Tobacco Policy Model. Preventive Medicine. 2001;33(6):558-70.
Chaiton M, Diemert L, Cohen JE, Bondy SJ, Selby P, Philipneri A, et al. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open. 2016;6(6):e011045.
Funding
Funding support for the parent clinical trial was provided by NIDA R01 DA021619 (PI: Carpenter). Additional support was provided by NIDA K23 DA045766 (Dahne) and NCATS UL TR001450.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Dr. Carpenter has received consulting honoraria from Pfizer, the maker of Chantix® (varenicline).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 189 kb)
Rights and permissions
About this article
Cite this article
Chen, B., Silvestri, G.A., Dahne, J. et al. The Cost-Effectiveness of Nicotine Replacement Therapy Sampling in Primary Care: a Markov Cohort Simulation Model. J GEN INTERN MED 37, 3684–3691 (2022). https://doi.org/10.1007/s11606-021-07335-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-021-07335-x