Abstract
Objective
This study aimed to explore the clinical value of adjuvant chemotherapy (ACT) following concurrent chemo-radiotherapy (CCRT) and induction chemotherapy (ICT) in loco-regionally advanced nasopharyngeal carcinoma (LANC).
Methods
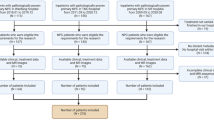
We included 839 newly diagnosed LANC patients in this study. ICT plus CCRT (ICT + CCRT group) was administered to 443 patients, and 396 patients received ACT after ICT plus CCRT (ICT + CCRT + ACT group). Univariate and multivariate Cox regression analyses were carried out. Furthermore, propensity score matching (PSM) was applied to balance the study and control groups.
Results
A total of 373 pairs of LANC patients were obtained after PSM analysis. We found that ACT following ICT + CCRT has no significant effect on improving the survival of LANC patients. By further exploring the ICT + CCRT + ACT treatment protocol, we excluded N0–1-positive patients and re-performed PSM in the ICT + CCRT and ICT + CCRT + ACT groups. Each group consisted of 237 patients. Kaplan–Meier analysis revealed that there were differences between the ICT + CCRT and ICT + CCRT + ACT groups in terms of the 5-year overall survival (OS) (78.9% vs. 85.0%, P = 0.034), disease-free survival (DFS) (73.4% vs. 81.7%, P = 0.029), and distant metastasis-free survival (DMFS) (84.9% vs. 76.0%, P = 0.019). In addition, the ICT + CCRT + ACT group had a higher incidence of grade 3/4 acute leukocytopenia/neutropenia.
Conclusion
Compared with ICT + CCRT, ACT following ICT plus CCRT can reduce distant metastasis of N2–3-positive LANC and improve the OS and DFS. The results demonstrated the feasibility and clinical utility of ACT following ICT plus CCRT.



Similar content being viewed by others
References
Afqir S, Ismaili N, Errihani H (2009) Concurrent chemoradiotherapy in the management of advanced nasopharyngeal carcinoma: current status. J Cancer Res Ther 5(1):3–7. https://doi.org/10.4103/0973-1482.48763
Al-Sarraf M, LeBlanc M, Giri PG et al (1998) Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 16(4):1310–1317. https://doi.org/10.1200/jco.1998.16.4.1310
Austin PC (2013) The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 32(16):2837–2849. https://doi.org/10.1002/sim.5705
Chan AT, Leung SF, Ngan RK et al (2005) Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 97(7):536–539. https://doi.org/10.1093/jnci/dji084
Chan ATC, Hui EP, Ngan RKC et al (2018) Analysis of plasma Epstein-Barr virus DNA in nasopharyngeal cancer after chemoradiation to identify high-risk patients for adjuvant chemotherapy: a randomized controlled trial. J Clin Oncol 15:10. https://doi.org/10.1200/JCO.2018.77.7847
Chen QY, Wen YF, Guo L et al (2011) Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst 103(23):1761–1770. https://doi.org/10.1093/jnci/djr432
Chen L, Hu CS, Chen XZ et al (2012) Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 13(2):163–171. https://doi.org/10.1016/s1470-2045(11)70320-5
Chen L, Hu CS, Chen XZ et al (2017) Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase 3 multicentre randomised controlled trial. Eur J Cancer 75:150–158. https://doi.org/10.1016/j.ejca.2017.01.002
Chen YP, Tang LL, Yang Q et al (2018) Induction chemotherapy plus concurrent chemoradiotherapy in endemic nasopharyngeal carcinoma: individual patient data pooled analysis of four randomized trials. Clin Cancer Res 24(8):1824–1833. https://doi.org/10.1158/1078-0432.CCR-17-2656
Chen YP, Chan ATC, Le QT et al (2019) Nasopharyngeal carcinoma. The Lancet 394(10192):64–80. https://doi.org/10.1016/s0140-6736(19)30956-0
Chen Y-P, IsmailaChua NMLK et al (2021a) Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO Guideline. J Clin Oncol 39(7):840–859. https://doi.org/10.1200/JCO.20.03237
Chen YP, Liu X, Zhou Q et al (2021b) Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. The Lancet 398(10297):303–313. https://doi.org/10.1016/s0140-6736(21)01123-5
Goto Y, Kodaira T, Fuwa N et al (2013) Alternating chemoradiotherapy in patients with nasopharyngeal cancer: prognostic factors and proposal for individualization of therapy. J Radiat Res 54(1):98–107. https://doi.org/10.1093/jrr/rrs071
He Y, Guo T, Wang J et al (2019) Which induction chemotherapy regimen followed by cisplatin-based concurrent chemoradiotherapy is the best choice among PF, TP and TPF for locoregionally advanced nasopharyngeal carcinoma? Ann Transl Med 7(5):104. https://doi.org/10.21037/atm.2019.02.15
Hui EP, Ma BB, Leung SF et al (2009) Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 27(2):242–249. https://doi.org/10.1200/JCO.2008.18.1545
Kim E, Matsuse M, Saenko V et al (2012) Imatinib enhances docetaxel-induced apoptosis through inhibition of nuclear factor-kappaB activation in anaplastic thyroid carcinoma cells. Thyroid 22(7):717–724. https://doi.org/10.1089/thy.2011.0380
Kwong KF, Edelman MJ et al (2005) High-dose radiotherapy in trimodality treatment of Pancoast tumors results in high pathologic complete response rates and excellent long-term survival. J Thorac Cardiovasc Surg 129(6):1250–1257. https://doi.org/10.1016/j.jtcvs.2004.12.050
Li WF, Chen NY, Zhang N et al (2019) Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: Long-term results of phase 3 randomized controlled trial. Int J Cancer 145(1):295–305. https://doi.org/10.1002/ijc.32099
Lin JC, Jan JS, Hsu CY et al (2003) Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 21(4):631–637. https://doi.org/10.1200/JCO.2003.06.158
National Cancer Institute-Common Toxicity Criteria Adverse Events Versions 4. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010–06 14_QuickReference_8.5x11.pdf.
National Comprehensive Cancer Network (2021) NCCN clinical practice guidelines in oncology: head and neck cancers. Version 2. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed Apr 2021
Ribassin-Majed L, Marguet S, Lee AWM et al (2017) What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol 35(5):498–505. https://doi.org/10.1200/JCO.2016.67.4119
Rusch VW, Giroux DJ, Kraut MJ et al (2007) Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 25(3):313–318. https://doi.org/10.1200/JCO.2006.08.2826
Song L, Liu H, Ma L et al (2013) Inhibition of autophagy by 3-MA enhances endoplasmic reticulum stress-induced apoptosis in human nasopharyngeal carcinoma cells. Oncol Lett 6(4):1031–1038. https://doi.org/10.3892/ol.2013.1498
Sun X, Su S, Chen C et al (2014) Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol 110(3):398–403. https://doi.org/10.1016/j.radonc.2013.10.020
Sun Y, Li WF, Chen NY et al (2016) Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 17(11):1509–1520. https://doi.org/10.1016/s1470-2045(16)30410-7
Tang LQ, Chen QY, Fan W et al (2013) Prospective study of tailoring whole-body dual-modality [18F]Fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol 31(23):2861–2869. https://doi.org/10.1200/jco.2012.46.0816
White AA, Lee DN, Mazzola E et al (2021) Adjuvant therapy following induction therapy and surgery improves survival in N2-positive non-small cell lung cancer. J Surg Oncol 123(2):579–586. https://doi.org/10.1002/jso.26305
Zhang X, Du L, Zhao F et al (2016) A phase II clinical trial of concurrent helical tomotherapy plus cetuximab followed by adjuvant chemotherapy with cisplatin and docetaxel for locally advanced nasopharyngeal carcinoma. Int J Biol Sci 12(4):446–453. https://doi.org/10.7150/ijbs.12937
Zhang Y, Chen L, Hu GQ et al (2019) Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med 381(12):1124–1135. https://doi.org/10.1056/NEJMoa1905287
Zou R, Yuan JJ, Li Q et al (2020) The clinical outcomes and toxicities of induction chemotherapy followed by concurrent chemoradiotherapy plus adjuvant chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Front Oncol 10:619625. https://doi.org/10.3389/fonc.2020.619625
Funding
No external funding was provided to support the writing of this article.
Author information
Authors and Affiliations
Contributions
R-HZ, Y-WY and H-YT conceived the study. HL, C-XH, RL, FH and H-YT collected all clinical data. H-YT and RL performed the statistical analyses. H-YT and K-PD prepared and edited the manuscript. All authors read and approved the final manuscript. Fang He has made contributions to the English language revision of this paper and put forward valuable modification comments.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare they have no competing interests.
Ethics approval
This retrospective study was approved by the Institutional Review Board (IRB) of the Affiliated Cancer Hospital & Institute of Guangzhou Medical University. Patients were required to provide written informed consent before enrolling in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tao, HY., Liu, H., He, F. et al. Adjuvant chemotherapy following combined induction chemotherapy and concurrent chemoradiotherapy improves survival in N2–3-positive nasopharyngeal carcinoma patients. J Cancer Res Clin Oncol 148, 2959–2969 (2022). https://doi.org/10.1007/s00432-021-03846-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03846-6